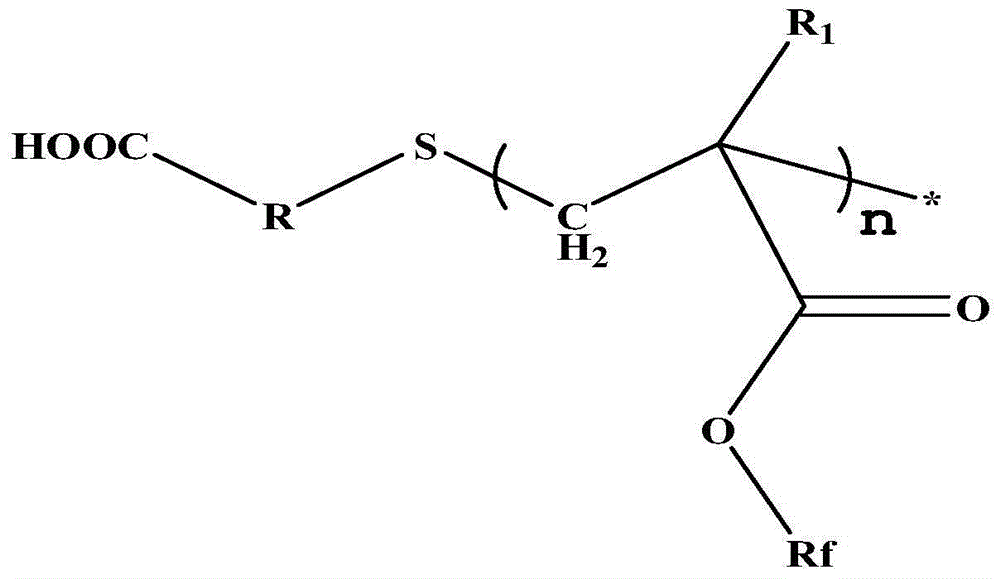
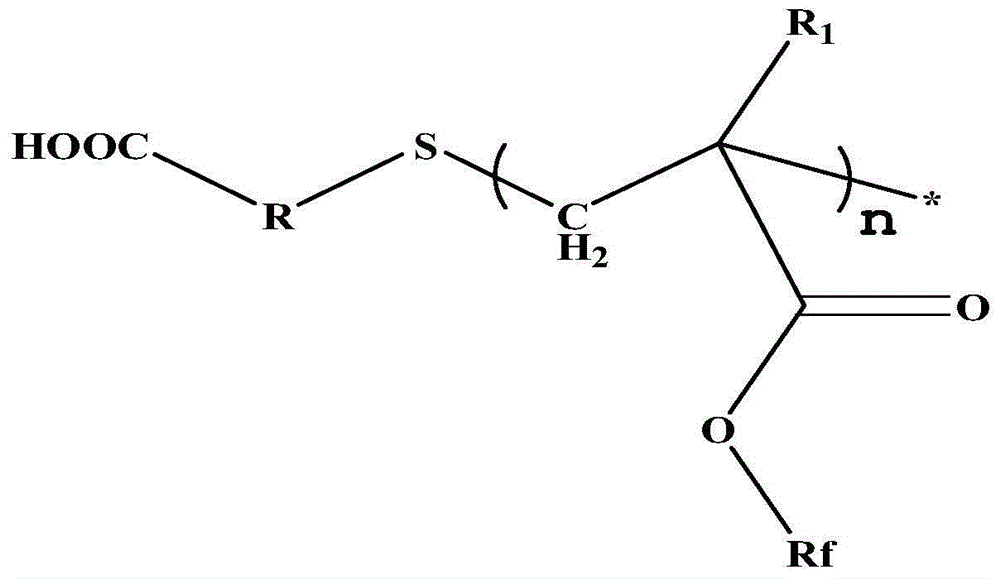
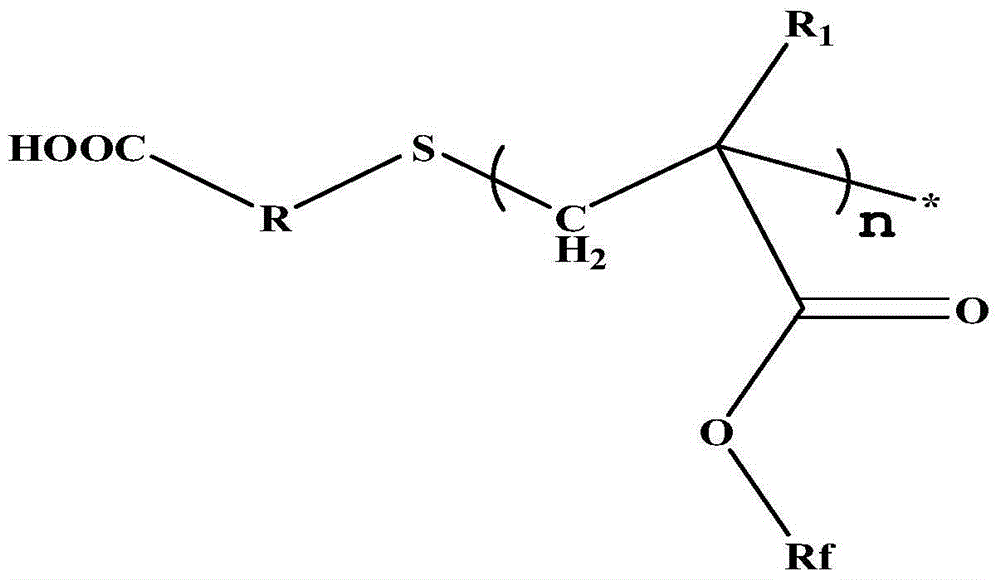
Carboxyl-terminated fluorine-containing prepolymer modified epoxy coating and preparation method and application thereof
A technology for epoxy coatings and prepolymers, applied in epoxy resin coatings, anti-corrosion coatings, coatings, etc., can solve problems such as poor compatibility, difficult migration of fluorine-containing groups, and difficulty in realizing molecular composites.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] (1) Preparation of carboxyl-terminated fluorine-containing prepolymer: Add 20 parts by mass of hexafluorobutyl methacrylate and 40 parts by mass of dioxane into a container, heat up to 85°C, add 1 part by mass of thioglycolic acid, azo 0.6 parts by mass of diisobutyronitrile was stirred and reacted for 7 hours to obtain a carboxyl-terminated fluorine-containing prepolymer solution, and the solvent was distilled off under reduced pressure to obtain a carboxyl-terminated fluorine-containing prepolymer. FT-IR (Bruker TENSOR27 (Germany) Fourier transform infrared spectrometer) at 3279~3658cm -1 with 1730cm -1 There are COOH and C=O absorption peaks respectively, 1 HNMR (deuterated DMSO as solvent) at 12.2ppm the proton absorption peak of COOH appeared, indicating the terminal carboxyl group of the product.
[0052] (2) Preparation of carboxyl-terminated fluorine-containing prepolymer modified epoxy coating: 10 mass parts of carboxyl-terminated fluorine-containing prepolym...
Embodiment 2
[0054] (1) Preparation of carboxyl-terminated fluorine-containing prepolymer: add 20 parts by mass of trifluoroethyl methacrylate and 40 parts by mass of butanone into a container, heat up to 78° C., add 4 parts by mass of 3-mercaptopropionic acid, 0.8 parts by mass of nitrogen diisoheptanonitrile was stirred and reacted for 6 hours to obtain a carboxyl-terminated fluorine-containing prepolymer solution, and the solvent was distilled off under reduced pressure to obtain a carboxyl-terminated fluorine-containing prepolymer. FT-IR at 3279~3658cm -1 with 1730cm -1 There are COOH and C=O absorption peaks respectively, 1 HNMR (deuterated DMSO as solvent) at 12.2ppm the proton absorption peak of COOH, indicating that the end of the product contains a carboxyl group.
[0055] (2) Preparation of modified epoxy coating with carboxyl-terminated fluorine-containing prepolymer: add 5 parts by mass of the carboxyl-terminated fluorine-containing prepolymer prepared in step (1) to 100 part...
Embodiment 3
[0057] (1) Preparation of carboxyl-terminated fluorine-containing prepolymer: Add 40 parts by mass of dodecafluoroheptyl methacrylate and 40 parts by mass of ethylene glycol dimethyl ether into a container, heat up to 85°C, and add 2-mercaptopropionic acid 0.6 parts by mass, 0.1 parts by mass of azobisisobutyronitrile, stirred and reacted for 8.5 hours to obtain a carboxyl-terminated fluorine-containing prepolymer solution, and the carboxyl-terminated fluorine-containing prepolymer was obtained after the solvent was distilled off under reduced pressure. FT-IR at 3266~3690cm -1 with 1735cm -1 There are COOH and C=O absorption peaks respectively, 1 HNMR (deuterated DMSO as solvent) at 12.2ppm the proton absorption peak of COOH, indicating that the end of the product contains a carboxyl group.
[0058] (2) Preparation of carboxyl-terminated fluorine-containing prepolymer modified epoxy coating: add 0.1 mass parts of carboxyl-terminated fluorine-containing prepolymer prepared in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com