Preparation method of immediate release oral preparation containing sitagliptin or sitagliptin pharmaceutical salt
An immediate-release preparation, the technology of sitagliptin, which is applied in the field of preparation of oral immediate-release preparations, can solve problems such as sticky punching, missing and incomplete tablet surfaces, and poor material compressibility, so as to ensure the therapeutic effect and solve sticky punching The effect of the phenomenon
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] prescription composition
[0068]
[0069] According to the above prescription, coated oral immediate-release tablets were prepared as follows:
[0070] Dissolving povidone K30 in purified water to prepare a binder solution with a weight percent content of 7.5%;
[0071] Sitagliptin phosphate, microcrystalline cellulose and anhydrous calcium hydrogen phosphate were mixed uniformly in a high-shear granulator to obtain a premix, wherein the stirring blade rotating speed was 180rpm, and the cutting knife rotating speed was 1500rpm; the granulation time was 10 minute;
[0072] Add the above binder solution to the above premix for wet granulation, sieve the soft material with a 1.0mm sieve for wet granulation, dry at 50°C for 2 hours, and then use a 1.0mm sieve for dry granulation to obtain particles;
[0073] Mix the croscarmellose sodium with the above-mentioned granules evenly (the rotation speed is 10rpm, mix for 15min), then add magnesium stearate and sodium stear...
Embodiment 2
[0078] prescription composition
[0079]
[0080] A coated oral immediate-release tablet was prepared in a similar manner to Example 1 except that the concentration of the binder solution was 7% by weight.
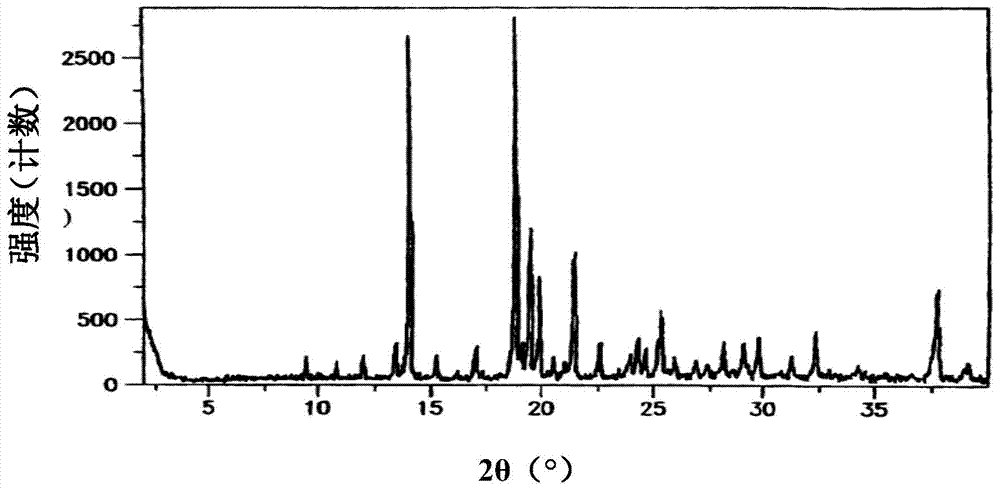
[0081] The quality of the oral immediate-release tablets prepared above was measured: the disintegration time limit was 26"-46", and there was no sticking phenomenon. Dissolution profile as Figure 8 As shown, the X-ray powder diffraction pattern is as Figure 9 As shown, it shows that the crystal form of sitagliptin phosphate hydrate has not changed.
Embodiment 3
[0083] prescription composition
[0084]
[0085] Except that no binder is used, the wetting agent is ethanol, and the granules are sized through a 1.5mm sieve, a method similar to that of Example 1 is used to prepare coated oral immediate-release tablets.
[0086] The quality of the oral immediate-release tablets prepared above was measured: the disintegration time limit was 27"-30", and there was no sticking phenomenon. Dissolution profile as Figure 10 As shown, the X-ray powder diffraction pattern is as Figure 11 As shown, it shows that the crystal form of sitagliptin phosphate anhydrous crystal form I has not changed. The fluidity of the total mixed powder obtained in this embodiment is worse than that of other embodiments, but still can meet the production requirements.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com