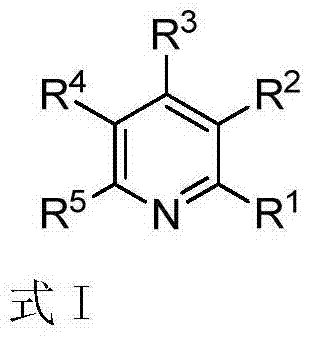
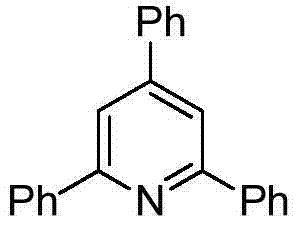
Polysubstituted pyridine derivative and preparation method thereof
A derivative and multi-substitution technology, applied in the field of multi-substituted pyridine derivatives and their preparation, can solve the problems of difficulty in synthesizing multi-substituted pyridine derivatives, difficult preparation of raw materials, limited use and the like, and achieves strong reaction specificity, The effect of post-processing green color and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Preparation of 2,4,6-triphenylpyridine
[0022]
[0023] Add 0.5mmol of 1,3-diphenyl-prop-2-yn-1-one, 0.5mmol of 1-phenylethylamine, 1mmol of potassium hydroxide, and 1.5mL of dimethyl sulfoxide into a 10mL reaction tube, and place In an oil bath at 100°C, react for 12h. Stop the reaction and cool to room temperature. The reaction solution was diluted with ethyl acetate, washed three times with water, and the organic phase was washed with anhydrous Na 2 SO 4 Dry, filter, concentrate, and purify by silica gel column chromatography to obtain 106.7 mg of the target product with a yield of 69%. The NMR characterization of this compound is as follows: 1 H NMR (400MHz, CDCl 3 )δ8.24–8.17(m,4H),7.87(s,2H),7.76–7.71(m,2H),7.53–7.40(m,9H); 13 C NMR (100MHz, CDCl 3 )δ157.5,150.16(s),139.6,139.0129.1,129.0,128.9,128.7,127.2,127.1,117.1.
Embodiment 2
[0025] Preparation of 2,6-diphenyl-4-(4-methylphenyl)pyridine
[0026]
[0027] Add 0.5mmol of 3-phenyl-1-(4-methylphenyl)-prop-2-yn-1-one, 0.5mmol of 1-phenylethylamine, 1mmol of potassium tert-butoxide, and 5mL of dimethyl sulfoxide into 10mL of In a reaction tube, place it in an oil bath at 80°C and react for 20 hours. Stop the reaction and cool to room temperature. The reaction solution was diluted with ethyl acetate, washed three times with water, and the organic phase was washed with anhydrous Na 2 SO 4 Dry, filter, concentrate, and purify by silica gel column chromatography to obtain 125.2 mg of the target product with a yield of 78%. The NMR characterization of this compound is as follows: 1 HNMR (400MHz, CDCl 3 )δ8.19(d, J=7.5Hz, 4H), 7.85(s, 2H), 7.62(d, J=8.0Hz, 2H), 7.50(t, J=7.5Hz, 4H), 7.43(d, J=7.3Hz, 2H), 7.30(d, J=7.9Hz, 2H), 2.41(s, 3H); 13 C NMR (100MHz, CDCl 3 )δ157.4, 150.0, 139.6, 139.0, 136.0, 129.8, 128.9, 128.6, 127.1, 126.9, 116.8, 21.2.
Embodiment 3
[0029] Preparation of 2,6-diphenyl-4-(2-tolyl)pyridine
[0030]
[0031] Add 0.5mmol of 3-phenyl-1-(2-methylphenyl)-prop-2-yn-1-one, 0.5mmol of 1-phenylethylamine, 1mmol of potassium tert-butoxide, and 3mL of dimethyl sulfoxide into 10mL of In a reaction tube, place it in an oil bath at 120° C., and react for 12 hours. Stop the reaction and cool to room temperature. The reaction solution was diluted with ethyl acetate, washed three times with water, and the organic phase was washed with anhydrous Na 2 SO 4 Dry, filter, concentrate, and purify by silica gel column chromatography to obtain 115.6 mg of the target product with a yield of 72%. The NMR characterization of this compound is as follows: 1 H NMR (400MHz, CDCl 3 )δ8.18(d, J=7.3Hz, 4H), 7.66(s, 2H), 7.49(t, J=7.5Hz, 4H), 7.42(t, J=7.2Hz, 2H), 7.32(d, J=5.3Hz, 4H), 2.35(s, 3H); 13 C NMR (100MHz, CDCl 3 )δ156.8, 151.3, 139.8, 139.5, 135.1, 130.7, 129.2, 129.0, 128.7, 128.3, 127.1, 126.1, 119.3, 20.4.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com