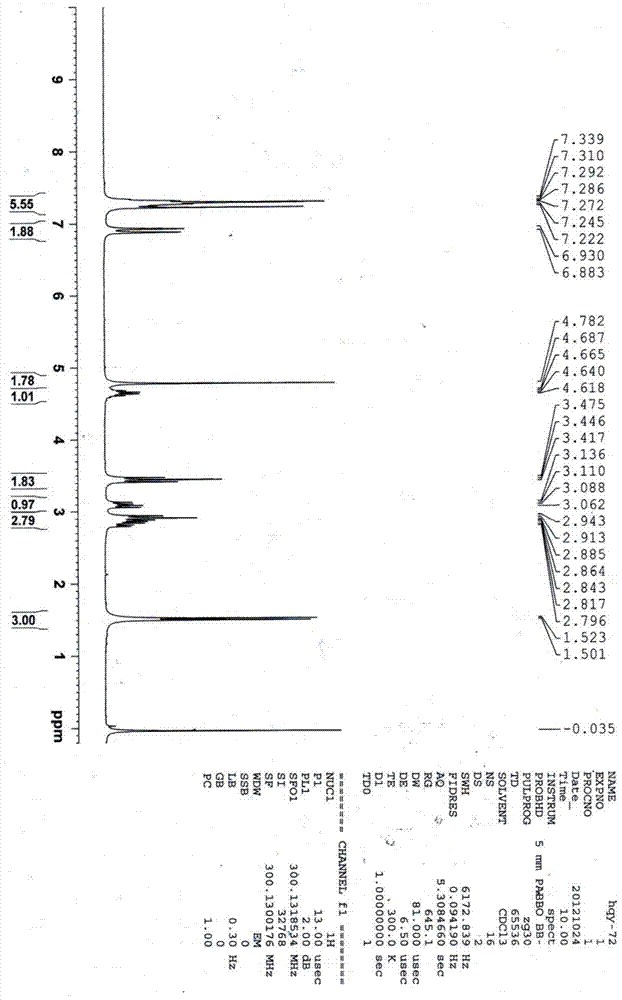
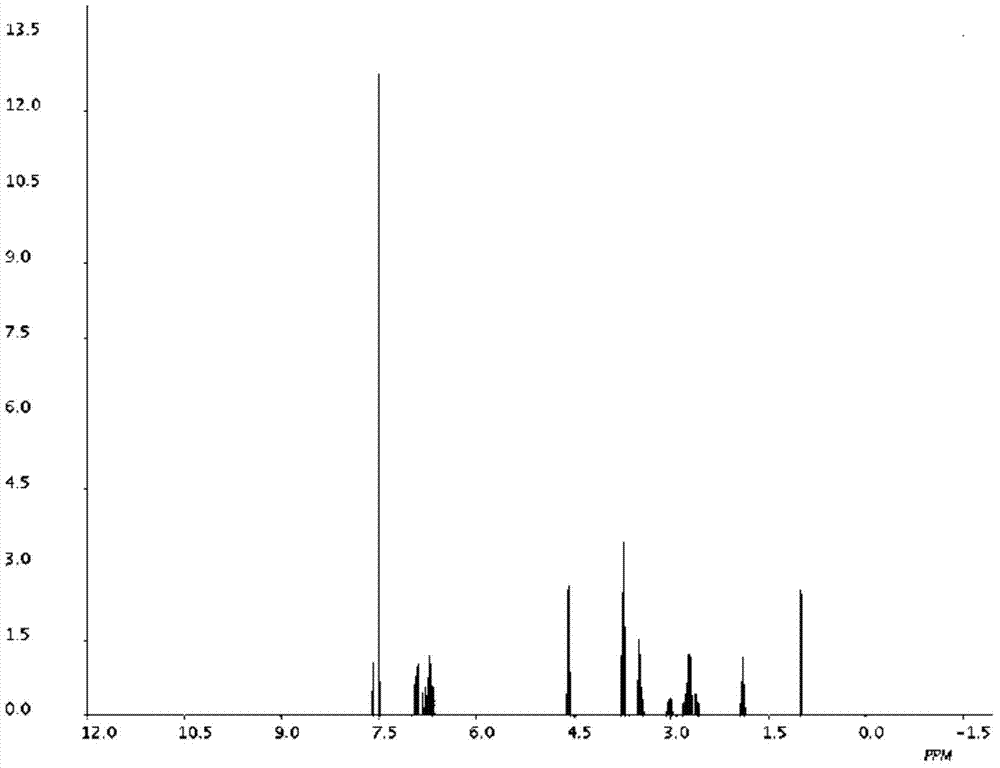
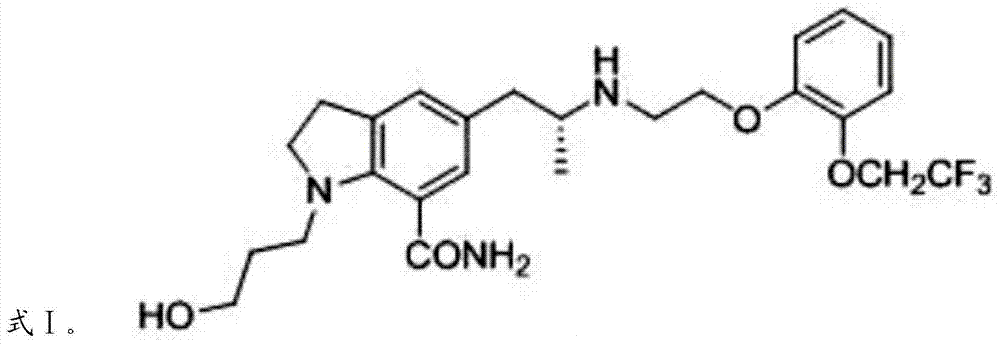
Silodosin intermediate, preparation method of silodosin intermediate and method for preparing silodosin from silodosin intermediate
A technology for silodosin and intermediates, which is applied in the field of compound synthesis and can solve the problems of multiple impurities, harsh purification conditions, low yield, high price of lithium tetrahydroaluminum and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0120] The preparation of the compound shown in embodiment 1 formula II
[0121] The preparation of compound shown in formula III:
[0122] raw material:
[0123] a. Benzyl chloride (M=126.5) 1265g (10.0mol)
[0124] b. Indoline (M=119.17) 596g (5.0mol)
[0125] c. Water 1Kg (55.5mol)
[0126] d. Sodium bicarbonate (M=84) 840g (10.0mol)
[0127] Feeding: a:b:d=2:1:2 (molar ratio), a, b reactants, c, d are catalysts.
[0128] Add a, c, and d into a 3L reaction flask, raise the temperature to 60-90°C, add b dropwise to the reaction solution, stir at 95°C for 1 hour after the drop, and detect by HPLC.
[0129] After the reaction, add 2L of toluene to the reaction solution, stir and separate layers, wash the organic layer once with 1L of water, dry, filter, and concentrate to obtain 1.17Kg (87.9%) of the compound represented by formula III.
[0130] The preparation of compound shown in formula IV:
[0131] raw material:
[0132] a. Dimethylformamide (M=73.09) 730.9Kg (10.0...
Embodiment 2
[0166] The preparation of embodiment 2 silodosin
[0167] The preparation of compound shown in formula VIII:
[0168] raw material:
[0169]
[0170] Feeding: a:e=1:45.6; a and e are reactants, b is catalyst, c and d are solvents.
[0171] Add a, c, d, and e into the reaction flask, stir for half an hour, then add b into it, after adding b, start to flow hydrogen, and after passing hydrogen for one minute, raise the temperature to 60°C for 1 hour, HPLC detection, raw materials After the reaction is complete, it can be processed.
[0172] After cooling the reaction liquid to room temperature, filter and remove the catalyst, add 150ml of ethyl acetate to the filtrate, adjust the pH to PH ≥ 8 with ammonia water, stir for half an hour, retest the pH, separate layers, and then use 100ml of acetic acid in the water layer Extracted once with ethyl ester, combined the organic layers, washed twice with 50ml of saturated brine, dried, filtered, and concentrated to obtain 1.96g of ...
Embodiment 3
[0188] Preparation of compound shown in embodiment 3 formula II
[0189] The preparation of compound shown in formula III:
[0190] raw material:
[0191] a. Benzyl chloride (M=126.5) 2530g (20.0mol)
[0192] b. Indoline (M=119.17) 596g (5.0mol)
[0193] c. Water 1Kg (55.5mol)
[0194] d. Sodium bicarbonate (M=84) 1680g (20.0mol)
[0195] Feeding: a:b:d=4:1:4 (molar ratio), a, b are reactants, c, d are catalysts.
[0196] Add a, c, and d into a 3L reaction bottle and raise the temperature to 60-90°C, add b dropwise to the reaction solution, stir at 95°C for 14 hours after the drop, and detect by HPLC.
[0197] After the reaction was completed, 2 L of toluene was added to the reaction liquid, the layers were stirred and separated, the organic layer was washed once with 1 L of water, dried, filtered and concentrated to obtain 1.17 Kg (purity 87.9%) of the compound represented by formula III.
[0198] The preparation of compound shown in formula IV:
[0199] raw material: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com