Preparation method of androst-4-ene-6beta,19-epoxy-3,17-dione
A technology of androsteroids and epoxy, applied in the direction of steroids, organic chemistry, etc., can solve the problems of expensive raw materials and the environment, and achieve the effects of low price, reduced environmental pollution, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
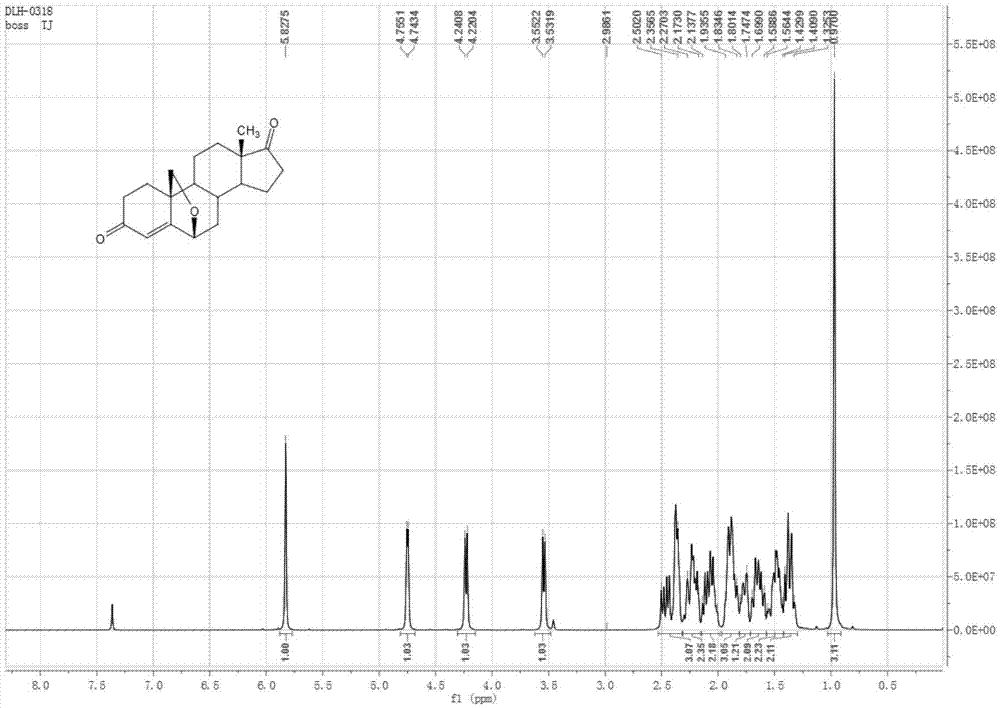
Image
Examples
Embodiment 1
[0047] Embodiment 1: the method for preparing androst-4-ene-6β,19-epoxy-3,17-dione
[0048] The by-product androst-5-ene-3α-hydroxyl-17-one produced in the production process of 19-norandrost-4-ene-3,17-dione is used as the starting material and protected at the 3-position , epoxidation, epoxy ring opening, 6,19-position ring closure / 3-position hydrolysis, oxidation-dehydration five-step reaction to prepare androst-4-ene-6β,19-epoxy-3,17-di ketone. Specifically include the following steps:
[0049] Step 1: 3-Bit Protection
[0050] Add 50 g of androst-5-ene-3α-hydroxyl-17-one, 250 ml of toluene, 50 ml of acetic anhydride, and 25 ml of pyridine into the single-necked bottle, and react under reflux for 3 to 5 hours. Recover toluene, add 300ml of water and stir for 30min, suction filter and dry to obtain 56g of androst-5-en-17-one-3α-ol acetate. Yield: 98%.
[0051] Step 2: Epoxidation
[0052] Add 50g androst-5-en-17-one-3α-ol acetate, 650ml dichloromethane, 150ml tert-but...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com