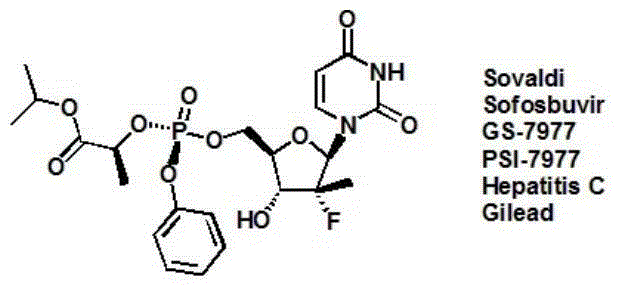
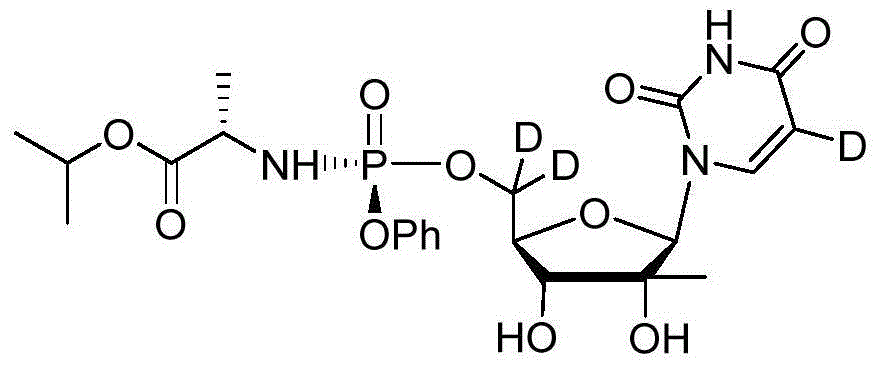
Deuterated Sofosbuvir and use thereof
A deuterated, deuterated alkyl technology, applied in the field of viral hepatitis C treatment drugs, can solve the problem of undisclosed chemical structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084]
[0085]
[0086] 1-[(2R,3R,4R,5R)-4-(tert-butyldimethylsilyloxy)-3-fluoro-5-hydroxymethyl-3-methyltetrahydrofuran-2-yl]pyrimidine— 2,4(1H,3H)-Diketone (212)
[0087]
[0088] Pyridine (30 mL) and dichloromethane (30 mL) were added to 211 (5.0 g, 19.21 mmol), and the solution was cooled to 0 °C. To the solution was added 4,4'-dimethoxytrityl chloride (7.16 g, 21.14 mmol), and the mixture was stirred at 0°C overnight. Methanol (5 mL) was added to quench the reaction, the reaction solution was concentrated to dryness under reduced pressure, and ethyl acetate (300 mL) and water (30 mL) were added to the residue. The organic layer was washed with brine (40 mL), dried over sodium sulfate. The solvent was removed under reduced pressure, and the residue was dissolved in dichloromethane (100 mL). To the solution was added imidazole (3.92 g, 57.5 mmol) and tert-butyldimethylsilyl chloride (4.34 g, 28.8 mmol). After the reaction mixture was stirred overnight at room ...
Embodiment 2
[0113]
[0114] Compound 217 (3 g) was dissolved in heavy water (30 mL), then deuterated Raney nickel (6 mL) was added and the reaction solution was stirred at 110° C. for 8 days. The reaction solution was cooled to room temperature, filtered through a thin layer of diatomaceous earth, and the oil obtained after the filtrate was concentrated was the product 218 (2.7 g, 90%).
[0115]
[0116] The dry compound 218 (2.7g) was dissolved in pyridine-dichloromethane (2:1, 40mL), benzoyl chloride (10mL) was slowly added at 0°C, and the reaction solution was then stirred at 0-4°C for reaction 4 After 1 hour, the reaction was quenched by pouring ice water, and the reaction mixture was concentrated under reduced pressure. The residue was partitioned between chloroform and saturated aqueous sodium bicarbonate solution, and the organic phase was washed with brine and dried. The crude product obtained after concentration was purified by column chromatography (hexane:ethyl acetate=4:...
Embodiment 3
[0126]
[0127] The mixture of 224 (930mg, 2.5mmol) and THF (13mL) was cooled to 0°C, and deuterated lithium borohydride (70mg, 2.71mmol) was added, then reacted at 0°C for 40 minutes, slowly raised to room temperature and continued stirring for one hour . After cooling to 0 °C, deuterated methanol was added to quench the reaction. The solvent was evaporated under reduced pressure, ethyl acetate (90mL) and water (10mL) were added to the residue, and the organic layer was washed with 1N hydrochloric acid solution (10mL), saturated sodium bicarbonate solution (20mL) and saturated brine (30mL), respectively. Dry over sodium sulfate and distill off the solvent under reduced pressure. Add 15 mL of dichloromethane to the obtained oily crude product and cool to 0°C, add triethylamine (0.68 mL, 5 mmol), slowly add MsCl (0.23 mL, 3.0 mmol) dropwise, slowly warm to room temperature and continue stirring for two hours. The reaction was quenched by adding 60 mL of dichloromethane and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com