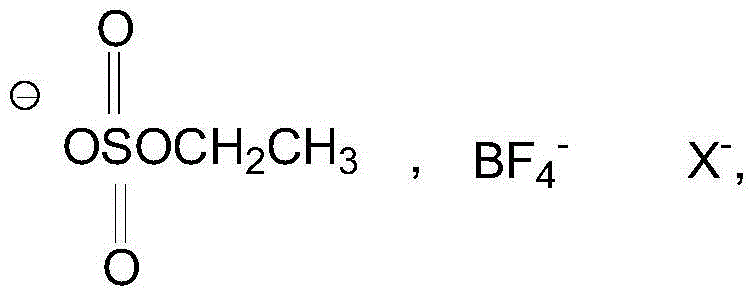Method for synthesizing imidacloprid employing cascade reaction
A technology of series reaction and imidacloprid is applied in the field of synthesis technology of insecticide imidacloprid, and can solve the problems of low purity affecting the quality of final product, many by-products, complicated steps and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
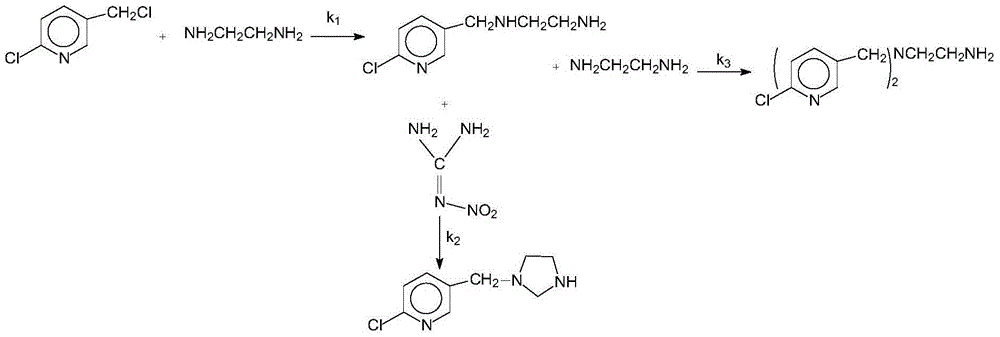
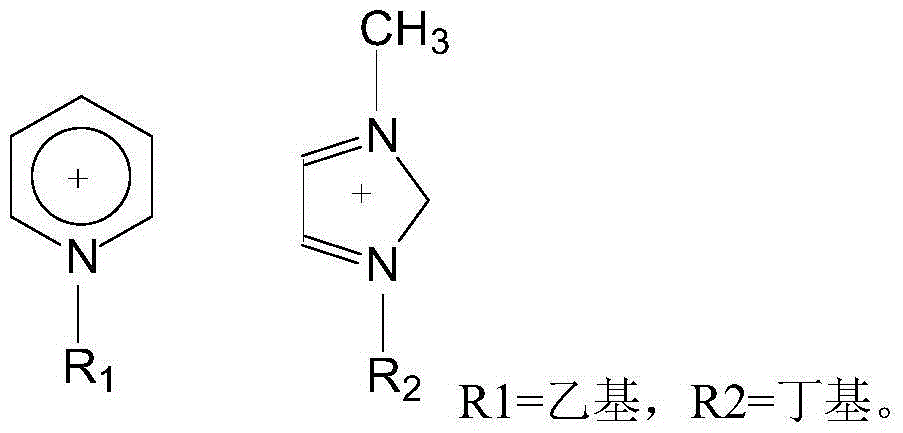
[0025] Add 1.62g (10mmol) 2-chloro-5-chloromethylpyridine, 1.04g (10mmol) nitroguanidine, 5ml ethanol solvent and 0.5g boron tetrafluoride 1-methyl-3-butyl in a three-necked flask Imidazole ionic liquid was added dropwise with 0.3ml of 37% hydrochloric acid, heated, and 3.01g (50mmol) of anhydrous ethylenediamine was added at 45°C with stirring, the pH was 5-6, and the heating was stopped after 3 hours of reaction. Add 1.5 g of potassium carbonate solid to remove HCl, and filter to obtain a yellow clear liquid. The ethanol solvent and unreacted anhydrous ethylenediamine were evaporated to obtain a pale yellow solid, that is, crude imidacloprid. The crude imidacloprid was added to pure water and heated and stirred to near boiling, filtered while hot, the obtained crystals were washed 3 times with pure water, the precipitate was dried and crystallized in acetone, and finally the crystals were vacuum dried to obtain 2.35 g of light yellow crystals. The purity was 98% by liquid ch...
Embodiment 2
[0027] Add 1.62g (10mmol) 2-chloro-5-chloromethylpyridine, 1.04g (10mmol) nitroguanidine, 6ml ethanol and 0.2g monoethyl hydrogen sulfate N-ethylpyridine ionic liquid into a three-necked flask, 0.3ml of 37% hydrochloric acid was added dropwise, heated, and 3.01g (50mmol) of anhydrous ethylenediamine was added at 45°C with stirring, the pH was 6-7, and the heating was stopped after 3 hours of reaction. Add 2 grams of potassium carbonate solid to remove HCl, and filter to obtain a yellow clear liquid. The ethanol solvent and unreacted anhydrous ethylenediamine were evaporated to obtain a pale yellow solid, that is, crude imidacloprid. The crude imidacloprid was added to pure water and heated and stirred to near boiling, filtered while hot, the obtained crystals were washed 3 times with pure water, the precipitate was dried and crystallized in acetone, and finally the crystals were vacuum dried to obtain 2.42 g of light yellow crystals. The purity was 96% by liquid chromatography...
Embodiment 3
[0029] Add 1.62g (10mmol) 2-chloro-5-chloromethylpyridine, 1.04g (10mmol) nitroguanidine, 6ml ethanol solvent and 0.2g monoethyl hydrogen sulfate N-ethylpyridine ionic liquid into a three-necked flask , And add dropwise 0.5ml of 37% hydrochloric acid, heat, add 3.01g (50mmol) of anhydrous ethylenediamine at 50°C under stirring, pH value is 4 to 5, after 3 hours of reaction, stop heating. Add 2 grams of potassium carbonate solid to remove HCl, and filter to obtain a yellow clear liquid. The ethanol solvent and unreacted anhydrous ethylenediamine were evaporated to obtain a pale yellow solid, that is, crude imidacloprid. The crude imidacloprid was added to pure water and heated and stirred to near boiling, filtered while hot, the obtained crystals were washed 3 times with pure water, the precipitate was dried and crystallized in acetone, and finally the crystals were dried under vacuum to obtain 2.52 g of light yellow crystals. The purity was 97% by liquid chromatography and the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com