Antitumor prodrug, activator, composition, and application thereof
An activator, anti-tumor technology, applied in the field of medicine, can solve the problems of drug toxicity not being well controlled, normal cell toxicity, human injury and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0222] Preparation of HK-1:
[0223]
[0224] 1. Add ethyl formate and ethyl sarcosinate hydrochloride (m / v=1 / 3, m is the mass of ethyl sarcosine hydrochloride) into the reaction flask at room temperature
[0225] 2. Add TEA into the bottle;
[0226] 3. Heat up to 50-55°C for reflux reaction for 8-10 hours;
[0227] 4. TLC central control: DCM / MeOH=10 / 1, PE / EA=2 / 1, iodine display, ninhydrin color development, UV;
[0228] 5. Cool to room temperature, filter out the solid, stir the filter cake with EA, combine the washings, and concentrate to dryness under reduced pressure at 40-50°C;
[0229] 6. After cooling, filter out the solid again, and weigh the filtrate to obtain yellow liquid HK-1 with a yield of ≥90%.
[0230] 1) Preparation of YS-1:
[0231]
[0232]
[0233] Step 1: Under nitrogen protection, add 590 g (4.5 mol) of HK-1 and 6 L of ethyl formate into a 10-liter four-necked flask, and add 200 grams of sodium hydride while stirring. After the addition was...
Embodiment 2
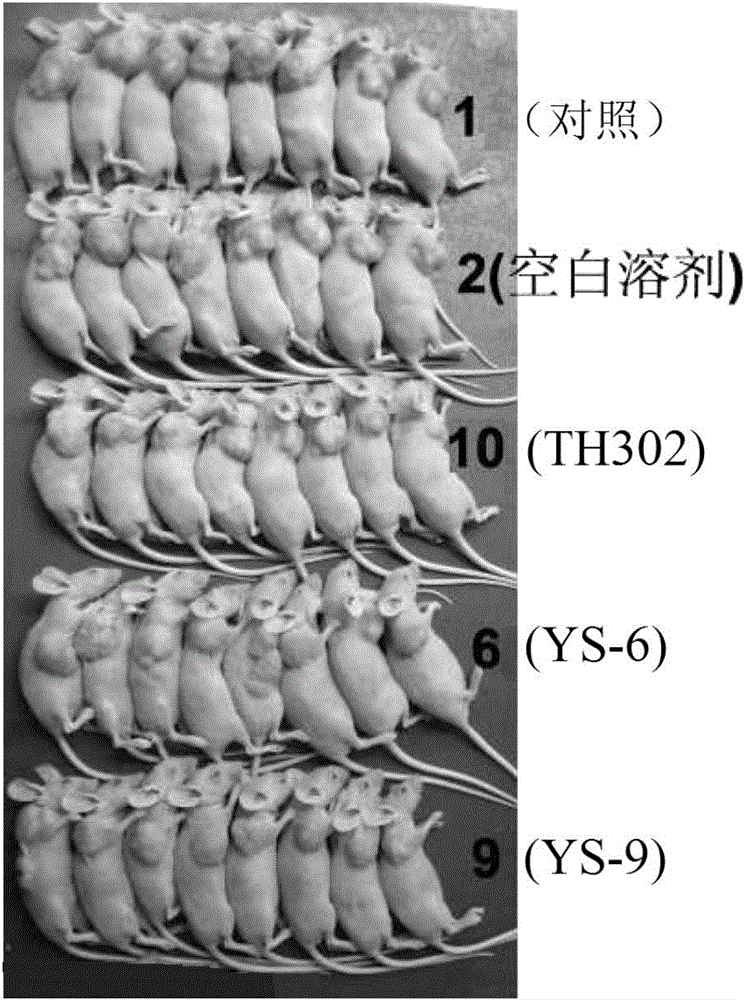
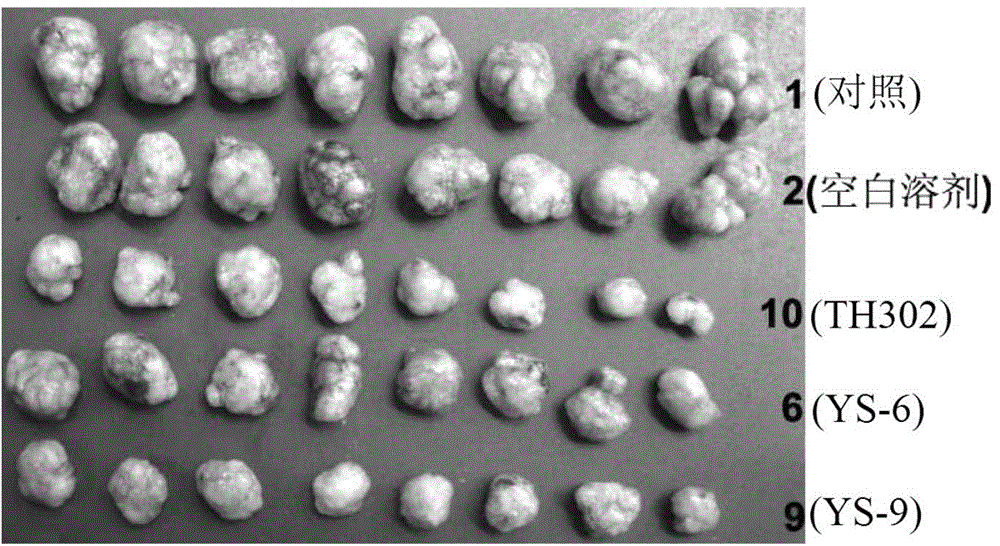
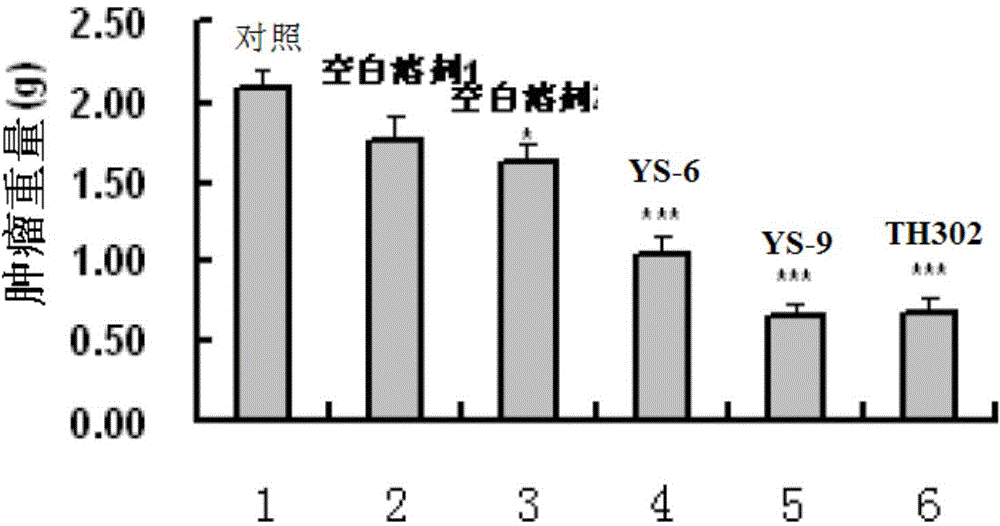
[0381] Embodiment 2: Activity experiment and result
[0382] Materials and Methods:
[0383] 1. Compound and preparation method: The present invention is numbered YS-6, YS-9, TH302, and the 5% DMSO+5% Tween-80 solution is prepared to the required concentration.
[0384] 2. Animals: nude mice (Balb / C nu / nu), 4-6 weeks old, purchased from Beijing Huafukang Biotechnology Co., Ltd., production license number: SCXK (Beijing) 2009-0004.
[0385] 3. Tumor cell line: Human lung adenocarcinoma cell line NCI-H460 (H460) is from the State Key Laboratory of Biotherapy, Sichuan University.
[0386] 4. Cell culture: H460 cells were cultured in RPMI 1640 medium (HyClone), which contained 10% fetal bovine serum (Hohhot Grassland Luye Bioengineering Materials Co., Ltd.) and 100U / ml penicillin and streptomycin. Take cells in the logarithmic growth phase, digest and count with 0.25% trypsin, and dilute the single cell suspension to 6×10 7 cells / ml for later use.
[0387] 5. Transplantation ...
Embodiment 3
[0419] Materials and Methods:
[0420] 1. Compounds and preparation methods: No. TH-302, YS-13, YS-14, YS-15, YS-16, 5% DMSO+5% Tween-80 solution prepared to the required concentration.
[0421] 2. Animals: nude mice (Balb / C nu / nu), 4-6 weeks old, purchased from Beijing Huafukang Biotechnology Co., Ltd., production license number: SCXK (Beijing) 2009-0004.
[0422] 3. Tumor cell line: Human lung adenocarcinoma cell line NCI-H460 (H460) is from the State Key Laboratory of Biotherapy, Sichuan University.
[0423] 4. Cell culture: H460 cells were cultured in RPMI 1640 medium (HyClone), which contained 10% fetal bovine serum (Hohhot Grassland Green Field Bioengineering Materials Co., Ltd.) and 100U / ml penicillin and streptomycin ( HyClone). Take cells in the logarithmic growth phase, digest and count with 0.25% trypsin, and dilute the single cell suspension to 6×10 7 cells / ml for later use.
[0424] 5. Transplantation and drug treatment:
[0425] Inoculation, grouping and tr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com