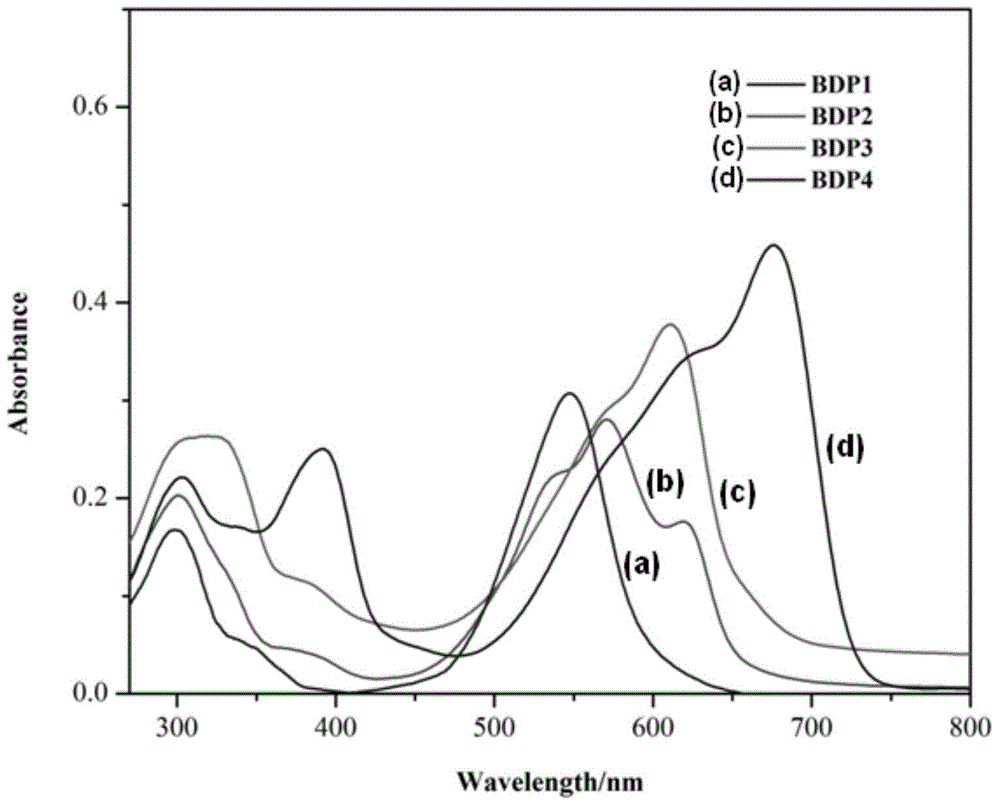
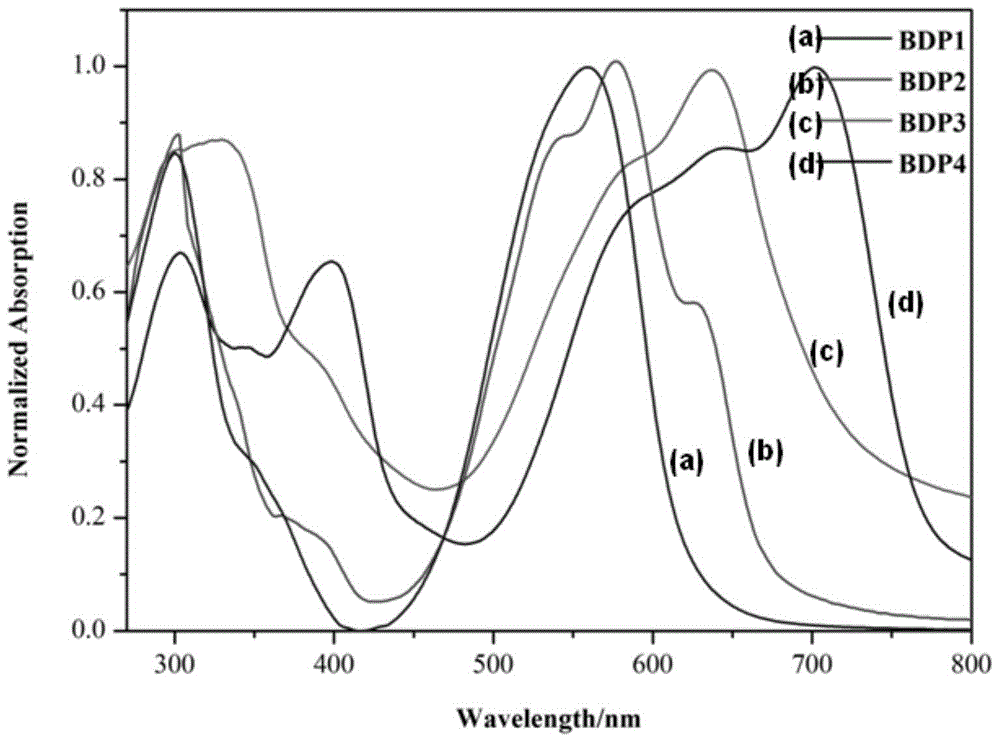
Meso-triphenylamine-substituted 3,5-aryl-modified boron dipyrromethene fluorophore derivatives and preparation method thereof
A technology of dipyrromethene and triphenylamine, which is applied in the field of organic solar cell materials, can solve the problems of low synthesis yield and achieve the effects of high product synthesis yield, low production cost, and easier control of reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Synthesis of BDP1
[0078]
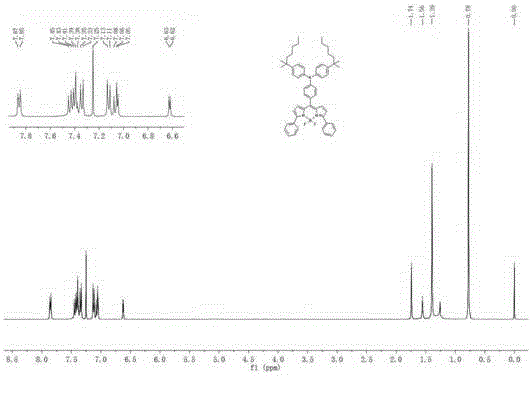
[0079]In a 100ml two-necked bottle, add intermediate 5 (200mg, 0.24mmol), 4,4,5,5-tetramethyl-2-phenyl-1,3,2-dioxaborane (118mg, 0.58mmol) , sodium carbonate (101.76 mg, 0.96 mmol), 15 ml toluene / water (V:V=1:1). Switch on the argon replacement air device for about 5 minutes, quickly add tetrakis(triphenylphosphine)palladium (11 mg, 0.01 mmol), and reflux at a constant temperature of 90°C for 24 hours. Stop the reaction, cool to room temperature, dilute with water, extract with anhydrous diethyl ether (3×20ml), wash the organic phase with saturated brine (3×30ml), dry over anhydrous magnesium sulfate, filter, spin off the solvent, and the crude product is filtered through silica gel The dye BDP1 was obtained by column chromatography with a yield of 73%. 1 H NMR (600MHz, CDCl 3 )δ7.86(d, J=8.0Hz, 4H), 7.44(d, J=8.7Hz, 2H), 7.40(d, J=7.5Hz, 4H), 7.34(dd, J=8.6Hz, 6H) ,7.12(d,J=8.6Hz,4H),7.05(d,J=4.4Hz,4H),6.62(d,J=4.2Hz,2H),1.74(s,4H),1...
Embodiment 2
[0081] Synthesis of BDP2
[0082]
[0083] The synthesis method of dye BDP2 is similar to the synthesis of dye BDP1. Intermediate 5 (200mg, 0.24mmol), furan-2-boronic acid (65mg, 0.58mmol), sodium carbonate (101.8mg, 0.96mmol), 15ml toluene / water (V:V=1:1). Switch on the argon replacement air device for about 5 minutes, quickly add tetrakis(triphenylphosphine)palladium (11 mg, 0.01 mmol), and reflux at a constant temperature of 90°C for 24 hours. Yield 68%. 1 H NMR (600MHz, CDCl 3 )δ7.72(d, J=3.1Hz, 2H), 7.57(d, J=1.5Hz, 2H), 7.37(d, J=8.6Hz, 2H), 7.33(d, J=8.6Hz, 4H) ,7.11(d,J=8.6Hz,4H),7.05(d,J=8.6Hz,2H),7.00(d,J=4.4Hz,2H),6.97(d,J=4.4Hz,2H),6.64 (d,J=3.6Hz,2H),1.74(s,4H),1.43-1.21(m,12H),0.78(s,18H). 13 C NMR (151MHz, CDCl 3 )δ150.32,146.77,146.27,145.36,144.27,143.87,136.55,131.89,129.72,127.32,126.31,124.99,119.64,118.18,115.09,113.36,57.18,38.35,32.45,31.76,31.44.MALDI-TOF-MS,m / z:calcd for C 51 h 56 BF 2 N 3 o 2 :791.440,found:791.418[M]+.
Embodiment 3
[0085] Synthesis of BDP3
[0086]
[0087] In a 100ml two-necked flask, dissolve Intermediate 5 (200mg, 0.24mmol) in 20ml of toluene, add 2-(tributyltin-based)thiophene (215mg, 0.58mmol), tetrakis(triphenylphosphine)palladium (11mg, 0.01mmol ), reacted at a constant temperature of 90°C for 24h. Stop the reaction, cool to room temperature, wash with saturated brine (3×30ml), extract with dichloromethane (3×40ml), dry over anhydrous sodium sulfate, filter, spin off the solvent, and the crude product is subjected to silica gel column chromatography to obtain the dye BDP3 , yield 65%. 1 H NMR (600MHz, CDCl 3 )δ8.17(d, J=3.8Hz, 2H), 7.46(d, J=5.9Hz, 2H), 7.37(2, J=6.9Hz, 1H), 7.33(d, J=8.6Hz, 4H) ,7.18(t,J=5.0Hz,2H),7.11(d,J=8.6Hz,4H),7.05(d,J=8.7Hz,2H),6.95(d,J=4.3Hz,2H),6.80 (d,J=4.3Hz,2H),1.74(s,4H),1.42-1.21(m,12H),0.77(s,18H). 13 C NMR (151MHz, CDCl 3 )δ150.39,149.31,146.35,143.83,134.52,131.95,130.85,129.96,128.94,128.63,127.34,125.06,120.18,119.53,77.23,77.02,76.81,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com