Method for preparing 6,6'-bi(2,3-dimethoxyphenyl)-alpha,alpha-D-trehalose and intermediates thereof
A technology of dimethoxybenzoyl and dimethoxybenzoic acid, which is applied in the field of preparation of 6,6'-bis-α,α-D-trehalose and its intermediates, can solve the problem that is not suitable for large-scale Production, difficult to remove triphenoxyphos, difficult to completely remove and other problems, to achieve the effect of increased yield, easy removal, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
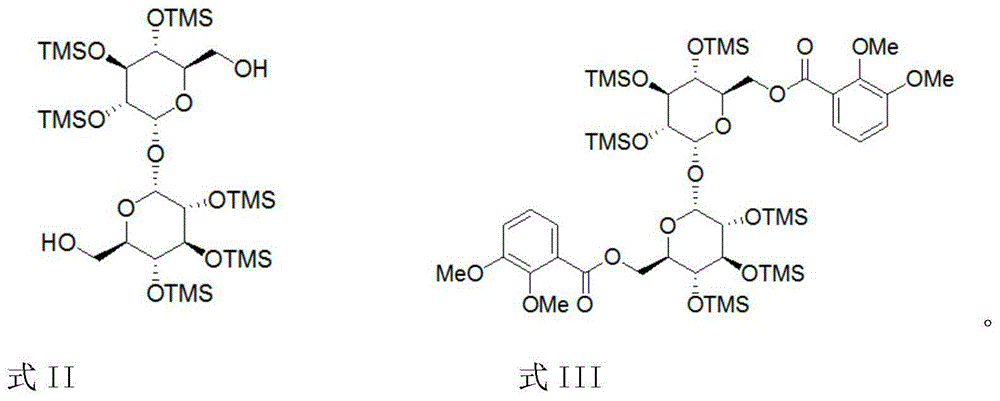
[0032] Example 1: Preparation of 6,6'-bis(2,3-dimethoxybenzoyl)-α,α-D-trehalose intermediate
[0033] Dissolve 5.46 g of 2,3-dimethoxybenzoic acid in 60 mL of anhydrous tetrahydrofuran, add 4.86 g of N,N'-carbonyldiimidazole, and stir at room temperature for 30 minutes. Add 7.75 g of the compound shown in II and 0.16 g of N-bromosuccinimide in a catalytic amount, and stir for 8 hours to react. Thin layer chromatography showed the reaction was complete. 60 mL of water was added to the reaction solution, stirred for 10 minutes, and extracted three times with 50 mL of dichloromethane. The organic layers were combined, washed three times with saturated potassium carbonate aqueous solution, then with saturated brine, and dried over anhydrous sodium sulfate. Filter and concentrate. Recrystallize the residue with 50 mL of ethanol / water (2 / 1) to obtain 10.3 g of the compound represented by formula III, which is 6,6'-bis(2,3-dimethoxybenzoyl)-α,α -D-trehalose intermediate, yield 93...
Embodiment 2
[0037] Example 2: Preparation of 6,6'-bis(2,3-dimethoxybenzoyl)-α,α-D-trehalose (scale-up test)
[0038] Dissolve 273 grams of 2,3-dimethoxybenzoic acid in 2.5 L of anhydrous tetrahydrofuran, add 243 grams of N,N'-carbonyldiimidazole, and stir at room temperature for 30 minutes. Add 388 grams of the compound shown in II and 8 grams of N-bromosuccinimide in a catalytic amount, and stir for 12 hours to react. Thin layer chromatography showed the reaction was complete. 2.5 L of water was added to the reaction solution, stirred for 10 minutes, and extracted three times with 1.25 L of dichloromethane. The organic layers were combined, washed three times with saturated potassium carbonate aqueous solution, then with saturated brine, and dried over anhydrous sodium sulfate. Filter and concentrate. The residue was recrystallized with 1.5 L of ethanol / water (2 / 1, volume ratio) to obtain 498 g of the compound represented by formula III, with a yield of 90%.
[0039] Dissolve 504 g o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com