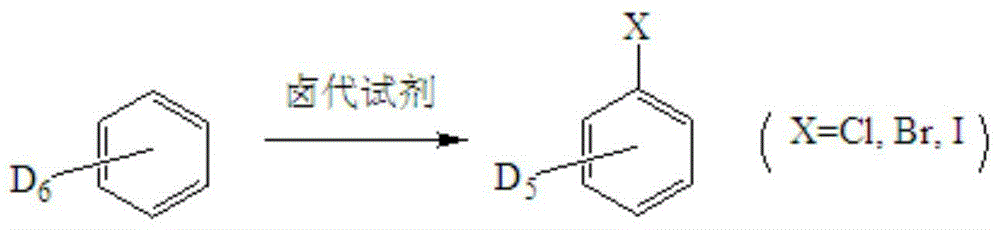
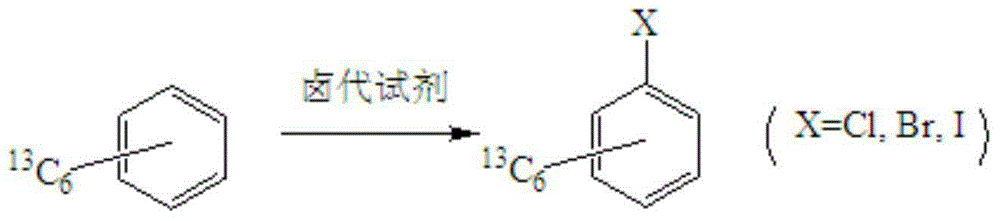
Synthetic method for stable isotope labeled halobenzene
A technology of stable isotopes and synthesis methods, which is applied in the field of synthesis of stable isotope-labeled halogenated benzenes, can solve the problems of expensive catalysts, strong corrosion, and high risk, and achieve good economy and use value, simple process, and low price. low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] A synthetic method for isotope D-labeled halogenated benzene, the method specifically comprises the following steps:
[0021] Mix isotope D-labeled benzene and phosphorus trichloride at a molar ratio of 1:1.1, CuCl and D-labeled benzene at a molar ratio of 1:1, and then place them in acetone. Control the reaction temperature at 90°C and pressure at 1Mpa, and stir the reaction After 12 hours, D-labeled chlorobenzene was obtained. The chemical purity of the obtained product was 99.4%, and the isotopic abundance was 99.0% atom D.
Embodiment 2
[0023] A synthetic method for isotope D-labeled halogenated benzene, the method specifically comprises the following steps:
[0024] Mix isotope D-labeled benzene and phosphorus pentachloride at a molar ratio of 1:1.0, CuI and D-labeled benzene at a molar ratio of 1.5:1, and then place them in dichloromethane. Control the reaction temperature at 60°C and pressure at 2Mpa. The reaction was stirred for 4 hours to obtain D-labeled chlorobenzene with a chemical purity of 99.2% and an isotope abundance of 99.2% atom D.
Embodiment 3
[0026] A synthetic method for isotope D-labeled halogenated benzene, the method specifically comprises the following steps:
[0027] The molar ratio of isotope D-labeled benzene to thionyl chloride is 1:1.5, Fe(NO 3 ) 3 Mix with D-marked benzene at a molar ratio of 0.1:1 and place in acetonitrile, control the reaction temperature at 110°C, pressure 4Mpa, and stir for 24 hours to obtain D-labeled chlorobenzene, which has a chemical purity of 99.2% and isotope-rich The degree is 99.3% atom D.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com