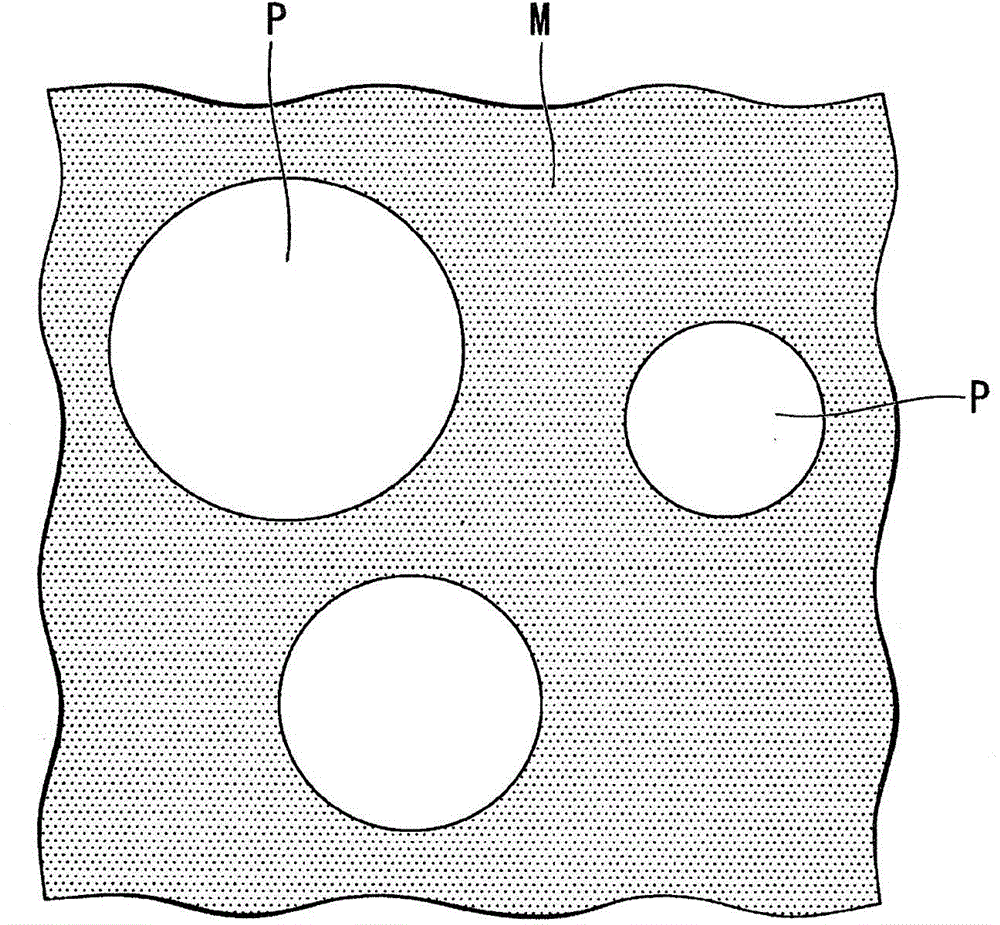
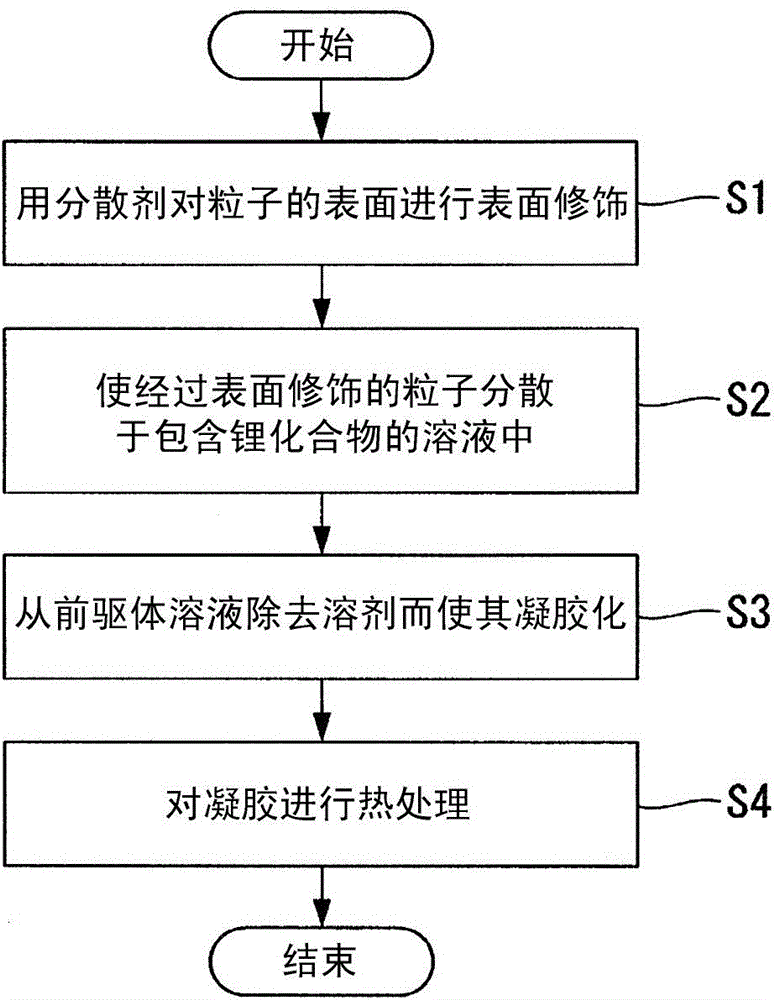
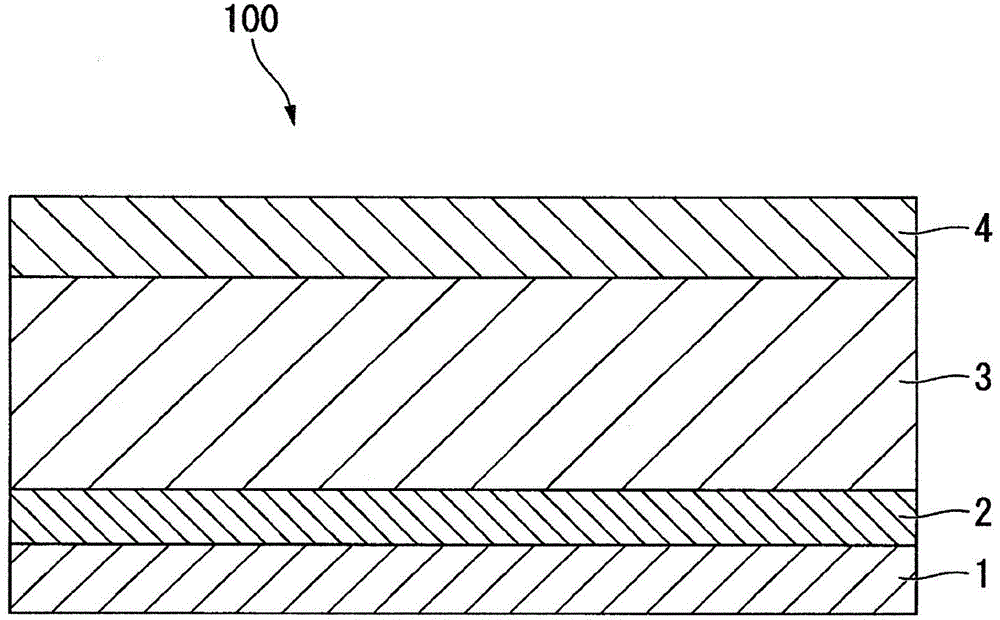
SOLID ELECTROLYTE, METHOD FOR PRODUCING SOLID ELECTROLYTE, AND LITHIUM-ION BATTEry
A technology of a solid electrolyte and a manufacturing method, which is applied to non-aqueous electrolyte storage batteries, electrolytes, lithium storage batteries, etc., can solve the problems of composition change and difficulty in manufacturing formed bodies.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0128] Using a planetary ball mill for Li 0.35 La 0.55 TiO 3 (High Purity Chemicals) pulverized to prepare particles with a volume fraction median diameter of 200 nm.
[0129] Next, add Li to 10ml of n-hexadecane 0.35 La 0.55 TiO 3 1 g of particles and 0.05 g of octadecyl monoethoxysilane (Azmax Co., Ltd.) were heated at 180° C. for 2 hours on a hot plate while stirring at 300 rpm using a magnetic stirrer.
[0130] Next, using a centrifuge cooled to 10° C., centrifugation was performed at 15,000 rpm for 10 minutes to settle the particles. After removing the supernatant, the particles were again dispersed in 10 ml of n-hexadecane, and centrifuged again under the same conditions. The supernatant was removed, and the obtained precipitate was dispersed in 10 ml of n-hexadecane to obtain a dispersion of surface-treated particles.
[0131] Next, 0.28 g of tetraethoxysilane (High Purity Chemical) was mixed with 10 ml of the obtained dispersion liquid. In addition, the LiNO 3...
Embodiment 2
[0135] As the particles, Li was synthesized with reference to the non-patent literature (Electrochemical and Solid-State Letters, 15(3)A37-A39(2012)).7 La 3 Zr 2 o 12 Except for the fine particles, a molded body of a solid electrolyte was obtained in the same manner as in Example 1.
Embodiment 3
[0147] Use 0.28g of octadecyltriethoxysilane, and add 4ml of silicone (KF-96-10cs, Shin-Etsu Silicone) and 3ml of indane instead of 0.28g of tetraethoxysilane (High Purity Chemical), and Except for this, a molded body of a solid electrolyte was obtained in the same manner as in Example 1.
[0148] The ionic conductivity of the obtained molded body of the solid electrolyte was measured in the same manner as in Example 1. As a result, the total ionic conductivity was 2.1×10 -4 S / cm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| ionic conductivity | aaaaa | aaaaa |
| ionic conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com