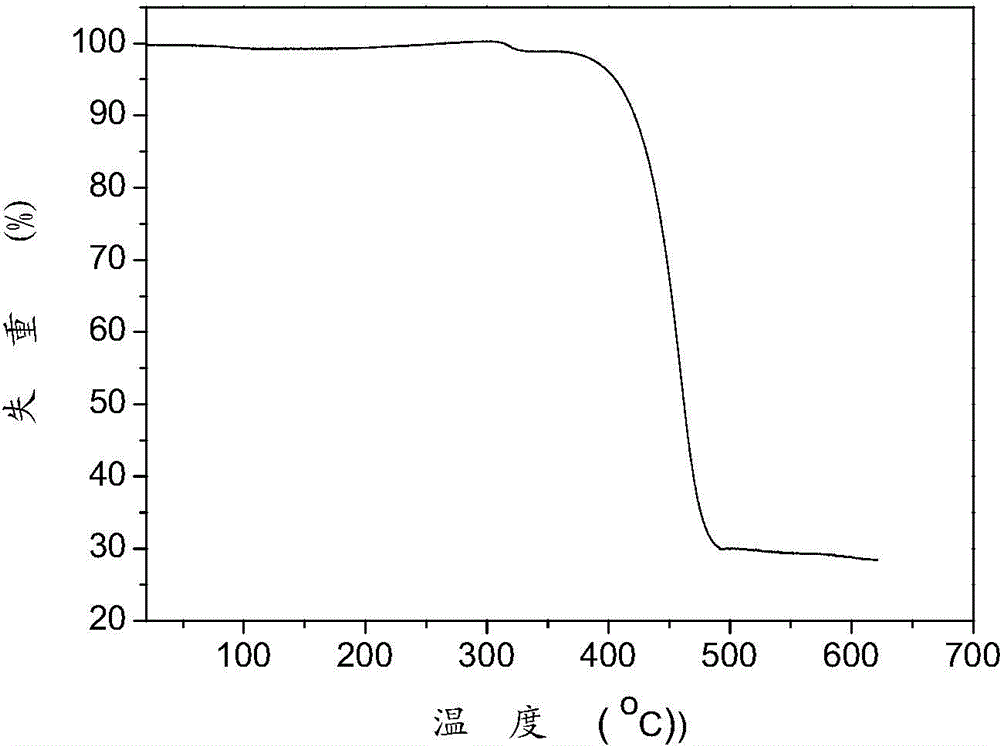
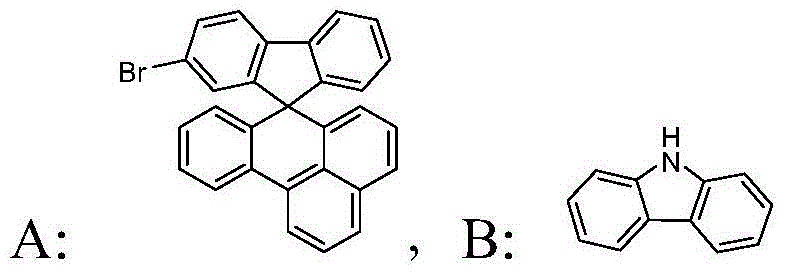A benzanthracene fluorenyl blue-light phosphorescent host material, a preparing method thereof and applications of the material
A benzanthracene fluorene-based, phosphorescent host technology, applied in the directions of luminescent materials, chemical instruments and methods, semiconductor/solid-state device manufacturing, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: The benzoanthracene fluorenyl blue phosphorescence host material in this example is 9-(spiro[benzo[de]anthracene-7,9'-fluorene]-2'-yl)-9H-carbazole, The structural formula is as follows:
[0027]
[0028] The preparation process of this compound is as follows:
[0029]
[0030] Under nitrogen protection, 2'-bromospiro[benzo[de]anthracene-7,9'-fluorene] (35.5g, 80mmol) was dissolved in 200mL N,N-dimethylformamide (DMF) solution, and then 9H-carbazole (33.3g, 80mmol), potassium carbonate (22.1g, 160mmol) and cuprous iodide (1.52g, 8mmol) were added to obtain a mixed solution, which was stirred and reacted at 120°C for 3 hours. Stop the reaction and cool to room temperature, filter, and wash the solid three times with distilled water. The crude product is separated by silica gel column chromatography using n-hexane as the eluent, and then dried under vacuum at 50°C for 24 hours to obtain an off-white solid benzanthracene fluorenyl blue phosphorescent host...
Embodiment 2
[0032] Example 2: The benzoanthracene fluorenyl blue phosphorescence host material in this example is 9-(spiro[benzo[de]anthracene-7,9'-fluorene]-2'-yl)-9H-carbazole, The structural formula is as follows:
[0033]
[0034] The preparation process of this compound is as follows:
[0035]
[0036] Under nitrogen protection, 2'-bromospiro[benzo[de]anthracene-7,9'-fluorene] (35.5g, 80mmol) was dissolved in 200mL toluene (Tol) solution, and then 9H-carbazole (14.7 g, 88mmol), cesium carbonate (57.2g, 176mmol), copper powder (0.768g, 12mmol) to obtain a mixed solution, which was stirred and reacted at 110°C for 6 hours. Stop the reaction and cool to room temperature, filter, and wash the solid three times with distilled water. The crude product is separated by silica gel column chromatography using n-hexane as the eluent, and then dried under vacuum at 50°C for 24 hours to obtain an off-white solid benzanthracene fluorenyl blue phosphorescent host material. The yield was 85%...
Embodiment 3
[0037] Example 3: The benzoanthracene fluorenyl blue phosphorescence host material in this example is 9-(spiro[benzo[de]anthracene-7,9'-fluorene]-2'-yl)-9H-carbazole, The structural formula is as follows:
[0038]
[0039] The preparation process of this compound is as follows:
[0040]
[0041] Under nitrogen protection, 2'-bromospiro[benzo[de]anthracene-7,9'-fluorene] (35.5g, 80mmol) was dissolved in 200mL acetonitrile (MeCN) solution, and then 9H-carbazole (16.0 g, 96mmol), potassium phosphate (39g, 184mmol), and cuprous oxide (2.3g, 16mmol) to obtain a mixed solution, which was stirred and reacted at 90°C for 8 hours. Stop the reaction and cool to room temperature, filter, and wash the solid three times with distilled water. The crude product is separated by silica gel column chromatography using n-hexane as the eluent, and then dried under vacuum at 50°C for 24 hours to obtain an off-white solid benzanthracene fluorenyl blue phosphorescent host material. The yield...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com