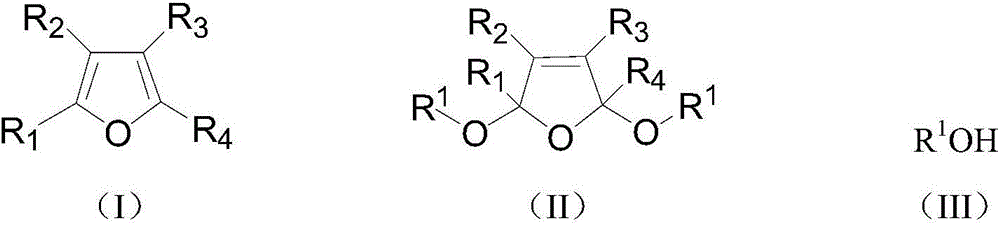
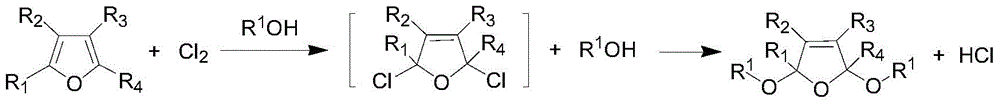
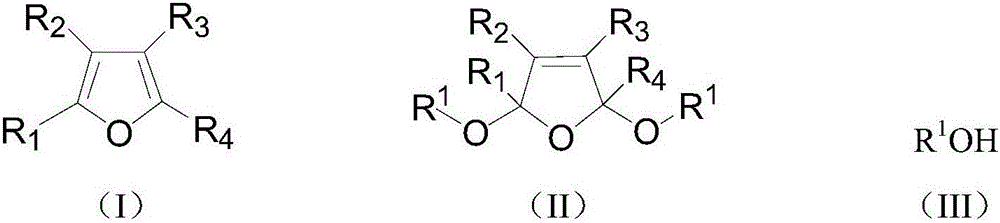
A kind of preparation method of 2,5-dialkoxy dihydrofuran compound
A dialkoxydihydrofuran and compound technology, which is applied in the field of preparation of 2,5-dialkoxydihydrofuran compounds, can solve the problems of low product yield, toxic solvent benzene, and low utilization rate of furan, etc. , to achieve the effect of simple reaction method, low production cost and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 60g of furan, 110g of sodium carbonate, 2g of phase-transfer catalyst methyltriethylammonium chloride, and 300g of methanol are put into the reactor, stirred, and when the temperature is lowered to -5°C to 5°C by opening a low-temperature constant temperature tank, 80g of chlorine gas is fed and kept at the Reaction temperature, reaction ends after reaction 10h. Remove residual chlorine in the reaction solution under reduced pressure, let it stand (making solid-liquid layering), filter (remove solid phase transfer catalyst and salts), add an appropriate amount of anhydrous sodium sulfate to the reaction solution after drying, and let it stand for a period of time After suction filtration (to remove sodium sulfate), the filtrate was distilled under reduced pressure to obtain 97.6 g of the product 2,5-dimethoxydihydrofuran with a yield of 85.3%.
Embodiment 2
[0027] 73g of 2-methylfuran, 170g of sodium bicarbonate, a total of 2g of methyl triethylammonium chloride and tetraethylphosphine hydroxide (mass ratio of 1:1) and 350g of methanol were added to the reactor, and stirred , when the temperature is lowered to -5°C to 5°C by opening a low-temperature thermostat, pass 80 g of chlorine gas and keep the reaction temperature, and the reaction ends after 10 hours of reaction. The post-treatment process was the same as in Example 1, and 107.3 g of the product 2-methyl-2,5-dimethoxydihydrofuran was obtained with a yield of 83.7%.
Embodiment 3
[0029] Put 85g of 2,5-dimethylfuran, 160g of pyridine, 2g of phase transfer catalyst dodecyltrimethylammonium chloride, and 400g of ethanol into the reactor, stir, and turn on the low-temperature thermostat to cool down to -5°C~5°C When, logical chlorine gas 80g and keep this reaction temperature, after reaction 10h, reaction finishes. The post-treatment process was the same as in Example 1, and 130.6 g of the product 2,5-dimethyl-2,5-diethoxydihydrofuran was obtained with a yield of 79.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com