Ceftiofur hydrochloride powder injection and its preparation method and application
A technology of ceftiofur hydrochloride and ceftiofur, which is applied in powder transportation, freeze-drying transportation, digestive system, etc., can solve the problems of ceftiofur sodium, such as strong hygroscopicity, easy to be degraded by moisture, easy to be degraded by moisture, etc., to achieve no Effects of incompatibility, increased feed intake, and broad market prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
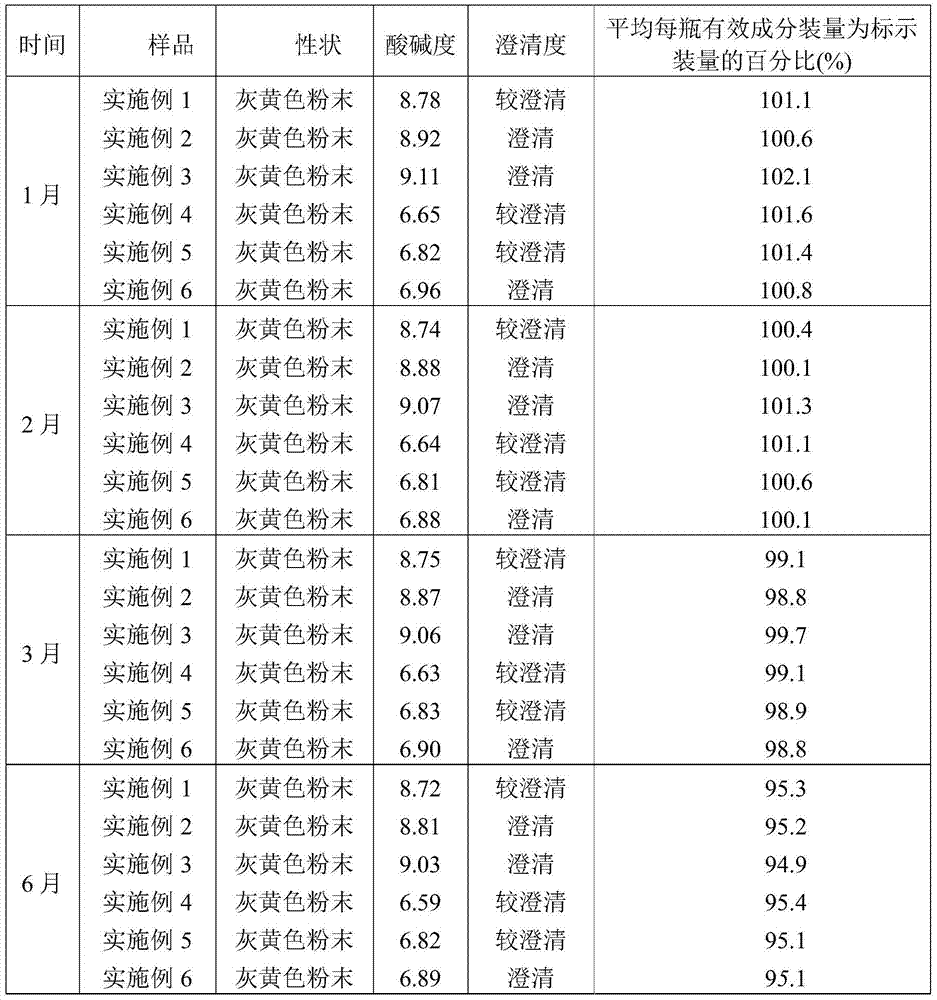
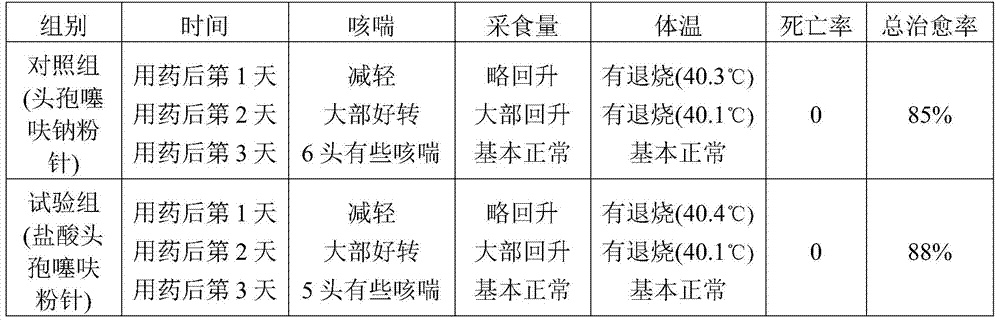
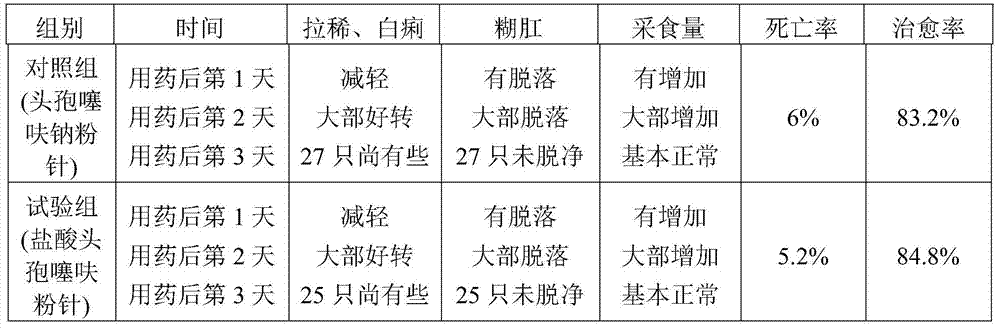
[0027] A preparation method of ceftiofur hydrochloride powder injection, comprising the following steps: weighing ceftiofur hydrochloride and sodium carbonate at a weight ratio of 48 (calculated as the active ingredient ceftiofur):28, and putting it into SYH-100 three-dimensional motion In the mixer, after mixing for 15 minutes, transfer to the KGL-120 type antibiotic glass bottle screw dispensing machine for subpackaging to obtain ceftiofur hydrochloride powder injection. The volume of each bottle is 0.1g, 0.2g, 0.5g or 1.0g (both based on the active ingredient ceftiofur), of which, 0.1g and 0.2g specifications are packed in 5-7mL vials, and 0.5g specifications are used in 10-15mL Divided into vials, 1.0g in 20-25mL vials.
[0028] The particle size of ceftiofur hydrochloride is 60-100 mesh.
[0029] The particle size of sodium carbonate is 60-100 mesh.
[0030] The above operations are all carried out in the powder injection purification workshop, and the cleanliness requi...
Embodiment 2
[0032] A preparation method of ceftiofur hydrochloride powder injection, comprising the following steps: weighing ceftiofur hydrochloride and sodium carbonate at a weight ratio of 50:31 (calculated as the active ingredient ceftiofur), and putting them into a SYH-100 three-dimensional motion In the mixer, after mixing for 15 minutes, transfer to the KGL-120 type antibiotic glass bottle screw dispensing machine for subpackaging to obtain ceftiofur hydrochloride powder injection. The volume of each bottle is 0.1g, 0.2g, 0.5g or 1.0g (both based on the active ingredient ceftiofur), of which, 0.1g and 0.2g specifications are packed in 5-7mL vials, and 0.5g specifications are used in 10-15mL Divided into vials, 1.0g in 20-25mL vials.
[0033] The particle size of ceftiofur hydrochloride is 60-100 mesh.
[0034] The particle size of sodium carbonate is 60-100 mesh.
[0035] The above operations are all carried out in the powder injection purification workshop, and the cleanliness r...
Embodiment 3
[0037] A preparation method of ceftiofur hydrochloride powder injection, comprising the following steps: weighing ceftiofur hydrochloride and sodium carbonate at a weight ratio of 52:34 (calculated as the active ingredient ceftiofur), and putting them into a SYH-100 three-dimensional motion In the mixer, after mixing for 15 minutes, transfer to the KGL-120 type antibiotic glass bottle screw dispensing machine for subpackaging to obtain ceftiofur hydrochloride powder injection. The volume of each bottle is 0.1g, 0.2g, 0.5g or 1.0g (both based on the active ingredient ceftiofur), of which, 0.1g and 0.2g specifications are packed in 5-7mL vials, and 0.5g specifications are used in 10-15mL Divided into vials, 1.0g in 20-25mL vials.
[0038] The particle size of ceftiofur hydrochloride is 60-100 mesh.
[0039] The particle size of sodium carbonate is 60-100 mesh.
[0040] The above operations are all carried out in the powder injection purification workshop, and the cleanliness r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com