A method of managing diabetic foot ulcers, pressure ulcers, venous leg ulcers and associated complication
A technology for diabetic foot ulcers, pressure ulcers, applied in the field of control of diabetic foot ulcers, pressure ulcers, venous lower extremity ulcers and related complications, can solve the problem of no suggestion or teaching to cure diabetic foot ulcers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1: the preparation of composition of the present invention
[0052] By blending with about 2% w / w microcrystalline cellulose, about 0.5% w / w polyvinylpolypyrrolidone and about 0.2% w / w magnesium stearate, the concentration range of about 55% w / w to about 99% w / w of A-type pentameric procyanidin flavonoids, trimers and tetramers of procyanidin flavonoids each in a concentration range of about 0.5% w / w to about 35% w / w of the present invention The composition is formulated as a capsule. This mixture is filled into capsules.
[0053] By adding suitable excipient(s) selected from the list comprising: granulating agents, binders, lubricants, disintegrants, sweeteners, glidants, anti-tacking agents, anti-adherent Static agents, surfactants, antioxidants, gums, coating agents, coloring agents, flavoring agents, coating agents, plasticizers, preservatives, suspending agents, emulsifying agents, plant cellulose materials and spheronizing agents or any combination ...
Embodiment 2
[0061] Embodiment 2: Composition of the present invention is effective in the diabetes mellitus induced by streptozotocin (STZ) with foot ulcer Evaluation of Diabetic Foot Ulcer Healing Activity in Rats
[0062] Induce diabetes in Sprague-Dawley rats (approximately 6-7 weeks old, approximately 180-200 g body weight) using the standard method of intraperitoneal (i.p) injection of streptozotocin (STZ) (approximately 50 mg / kg, i.p.) . After approximately 48 hours, rats with glucose levels greater than 300 mg / dl (indicative of diabetes) were selected for the study.
[0063] About 14 days after STZ administration, wounds were generated, and this day was defined as Day 0. A wound was created on the right hind paw (foot) of each rat. Each rat was anesthetized with an intraperitoneal injection of approximately 80 mg / kg ketamine. A rectangular pattern was marked on the dorsal surface of the right hind paw (foot) using a flexible transparent plastic template, and then a full-thic...
Embodiment 3
[0094] Example 3: Effects of Compositions of the Invention in Subjects Suffering from Diabetic Foot Ulcers
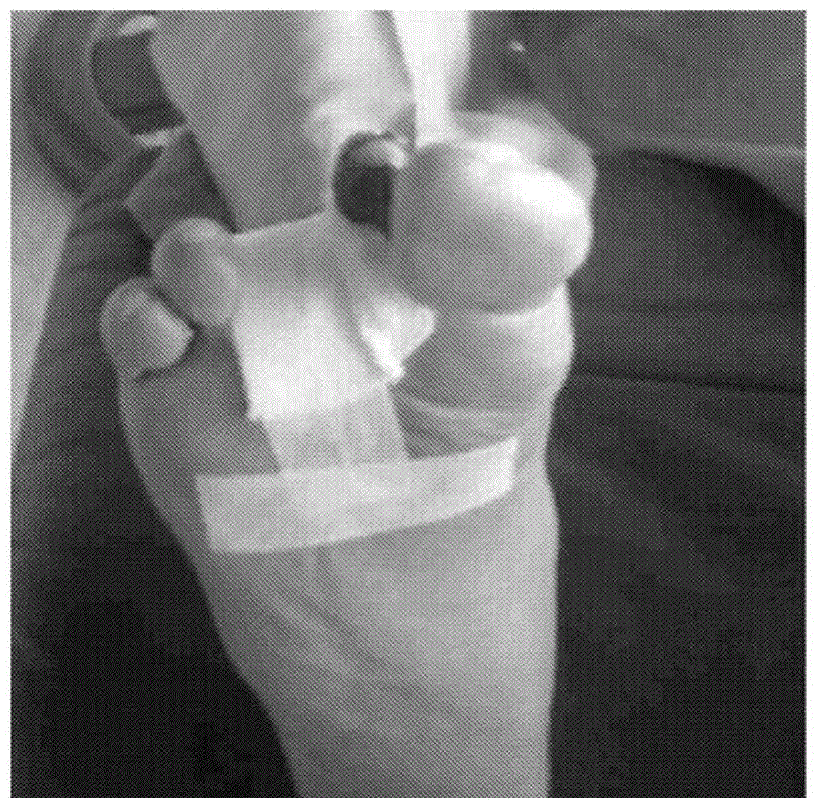
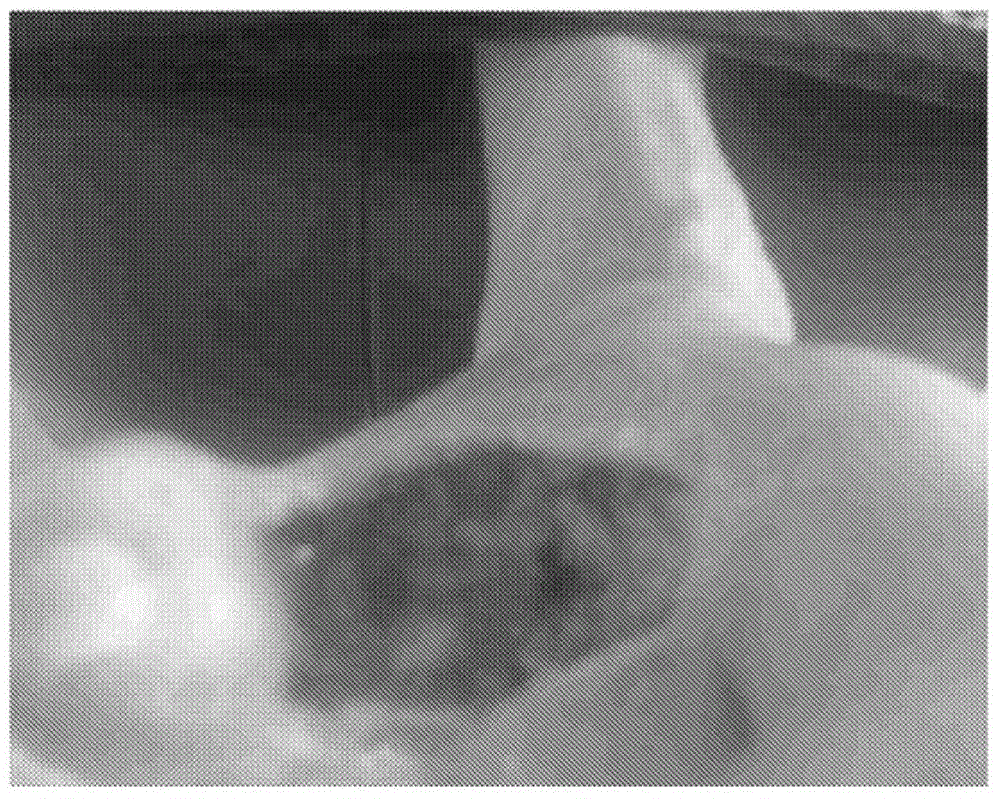
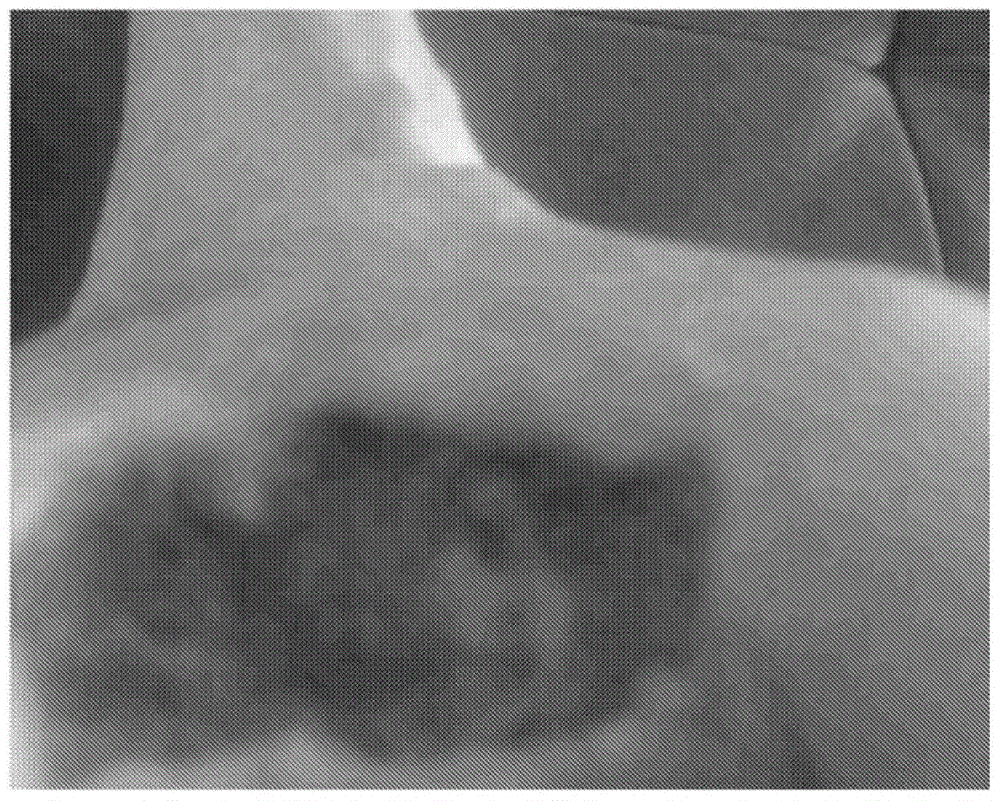
[0095] A study was conducted to evaluate the efficacy of the compositions of the invention in human subjects with diabetic foot ulcers. The selected subjects (male, age 85 years) were chronic diabetic patients diagnosed with type 2 diabetes for more than 25 years. The subject used insulin along with various oral antidiabetic medications. Subject developed a diabetic foot ulcer which resulted in the amputation of a gangrenous toe and two fingers ( figure 1 ).
[0096] The amputation wound further becomes a non-healing ulcer with a necrotic environment in the wound area. Subjects were at greater risk of further amputation at the start of the study in order to reduce the development of decay disease.
[0097] The subjects were administered capsules of the composition of the present invention at a dose of about 300 mg twice daily for a period of about 11 months, calcu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| depth | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com