Multiple real-time fluorescent quantative PCR rapid diagnosis kit for six porcine viruses
A real-time fluorescence quantitative and rapid diagnosis technology, applied in the determination/inspection of microorganisms, biochemical equipment and methods, etc., can solve the problems of difficult early detection, cumbersome operation, time-consuming and laborious, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Embodiment 1 kit structure
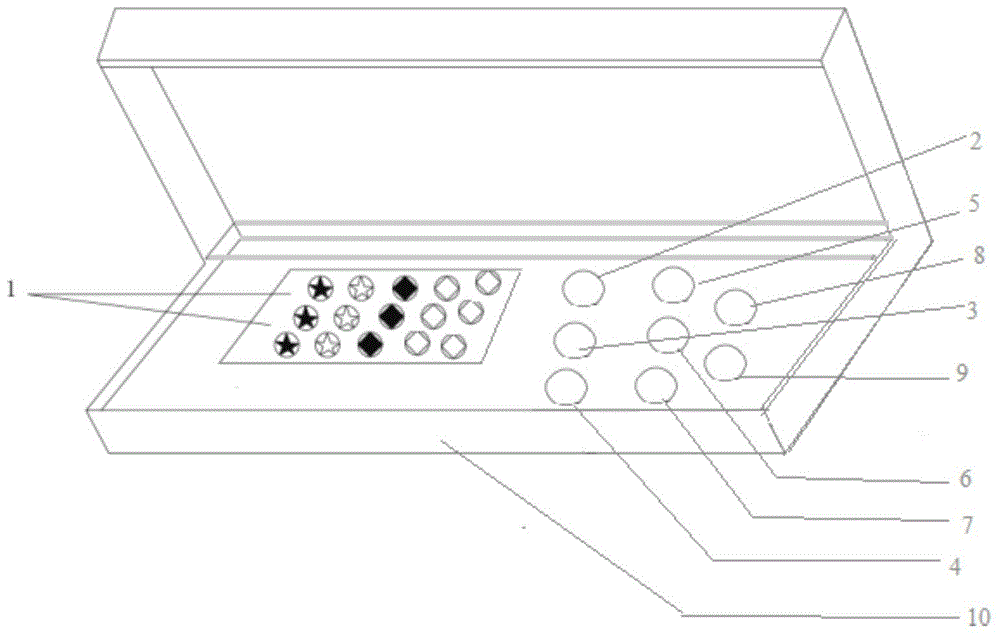
[0077] see figure 1 , a multiplex real-time fluorescent quantitative PCR rapid diagnostic kit for six porcine viruses, consisting of fluorescent quantitative PCR reaction solution 1, PCV2 virus standard product 2, PPV virus standard product 3, PRRSV virus standard product 4, CSFV virus standard product 5, PEDV virus Standard substance 6, PRV virus standard substance 7, positive control substance 8, negative control substance 9 and box body 10.
[0078] The box body 10 is provided with corresponding container holes for respectively placing quantitative PCR reaction solution tubes, PCV2 virus standard product tubes, PPV virus standard product tubes, PRRSV virus standard product tubes, CSFV virus standard product tubes, and PEDV virus standard product tubes. , PRV virus standard quality control, positive control quality control and negative control quality control. Wherein the fluorescent quantitative PCR reaction solution comprises 3 tubes o...
Embodiment 2
[0086] Embodiment 2 preparation of plasmid standard
[0087] Step 1: Primer Synthesis
[0088] The 6 viral primer sequences (see Table 1) designed by the present invention were synthesized in China Shanghai Sangon Biology Technology Service Company, and the synthesis amount was 2OD per primer.
[0089] Step 2: Total viral DNA / RNA extraction
[0090] Put 10ul of dry powder samples containing PCV2, PPV, PRRSV, CSFV, PEDV and PRV virus sources into 1.5ml centrifuge tubes, and use the MiniBEST Viral RNA / DNA Extraction Kit Ver. .4.0 (TAKARA) extraction to obtain viral RNA / DNA.
[0091] Step 3: Synthesize cDNA by reverse transcription
[0092] Reverse transcription reaction: Add 0.5 μl of the total RNA template prepared in the previous step to an RNase-free 0.2ml PCR tube, and perform RT- PCR reaction, after the reverse transcription reaction is completed, the resulting reaction solution is the cDNA / DNA template.
[0093] Step 4: Common PCR amplification
[0094] PCR reaction ...
Embodiment 3
[0097] Embodiment 3 The present invention detects six kinds of virus-specific experiments
[0098] Step 1: Primer Synthesis
[0099] The 6 viral primer sequences (see Table 1) designed by the present invention were synthesized in China Shanghai Sangon Biology Technology Service Company, and the synthesis amount was 2OD per primer.
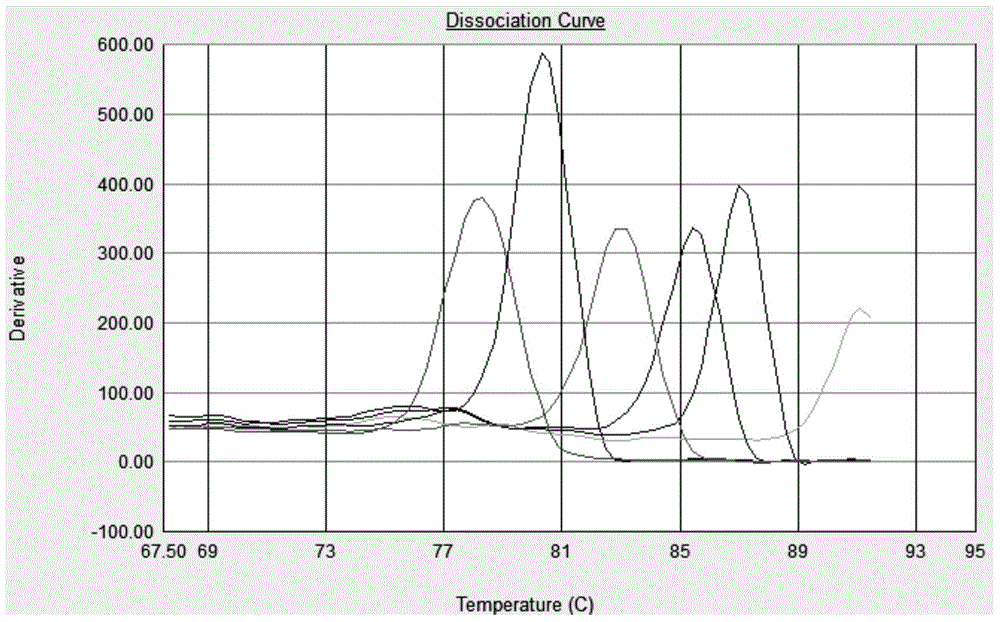
[0100] Step 2: Specific detection of a single virus in a multiplex system
[0101] According to the EvaGreen multiplex real-time fluorescent quantitative PCR reaction system, 6 identical reaction solutions were prepared, and 1 μl of 1.0×10 6 Copies / μl of PCV2, PPV, PRRSV, CSFV, PEDV and PRV plasmid standards, that is, the reaction system is 5μl of 10×PCR buffer, 25mM MgCl 2 5μl, 2.5mM dNTP 4μl, 20×EvaGreen 2.5μl, Ex Taq DNA polymerase 2.5U, in which the final concentrations of upstream and downstream primers for PCV2, PPV, PRRSV, CSFV, PEDV and PRV are 0.26, 0.12, 0.14, 0.13, 0.05 and 0.13 respectively μM, 1μl template, add ddH 2 O to the total...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Copy number | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com