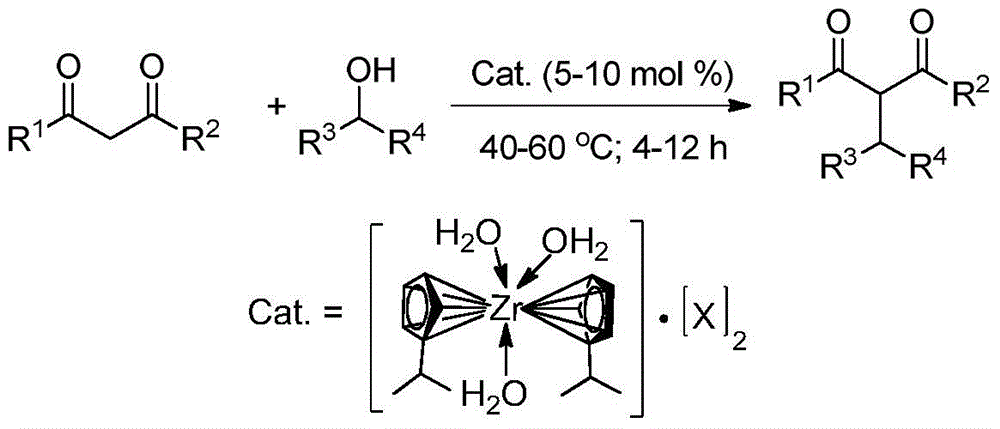
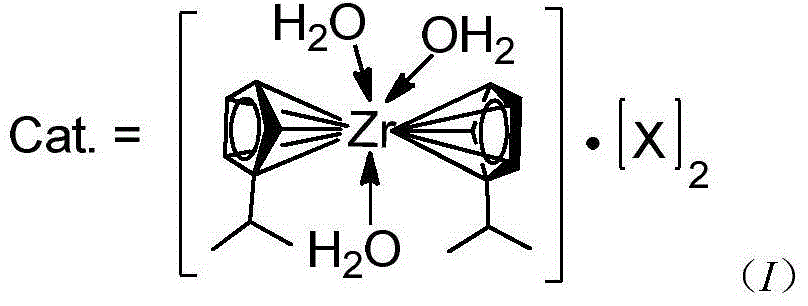
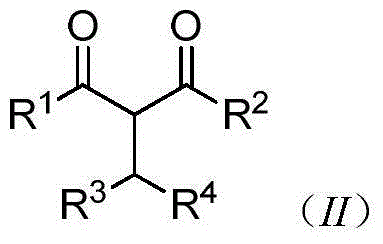
Preparation and application of novel isopropyl zirconocene complex
A technology of propyl zirconocene and complexes, which is applied in the field of green catalytic synthesis of 1,3-dicarbonyl compounds, can solve the problems of limited application, the need to react under anhydrous conditions, and the catalyst is easy to absorb water, etc. Stable, mild reaction conditions, wide range of substrate effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] The present invention will be further described below in conjunction with specific preparation examples: The present invention will be further described below in conjunction with specific preparation examples:
[0022] Preparation of perfluoroalkyl (aryl) sulfonate isopropyl zirconocene catalyst:
preparation example 1
[0024] Preparation of catalyst perfluoroalkyl (aryl) sulfonate isopropyl zirconocene: dichlorodiisopropyl zirconocene dissolved in THF, N 2 Under protection, a THF solution of silver perfluorooctane sulfonate was added, and stirred at room temperature in the dark for 1 h. After filtration, add n-hexane to the filtrate until the layers are separated. Put it in the refrigerator for 12 hours, and the solid of the complex was precipitated, and the yield was 60%.
preparation example 2
[0026] Catalyst Isopropyl zirconocene perfluorooctane sulfonate (X=OSO 2 C 6 f 5 ) preparation: dichlorodiisopropyl zirconocene was dissolved in acetonitrile, N 2 Under protection, add silver perfluorophenylsulfonate in acetonitrile solution, and stir at room temperature in the dark for 1.5h. After filtration, add n-hexane to the filtrate until the layers are separated. Put it in the refrigerator for 12 hours, and the complex solid was precipitated, with a yield of 61%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com