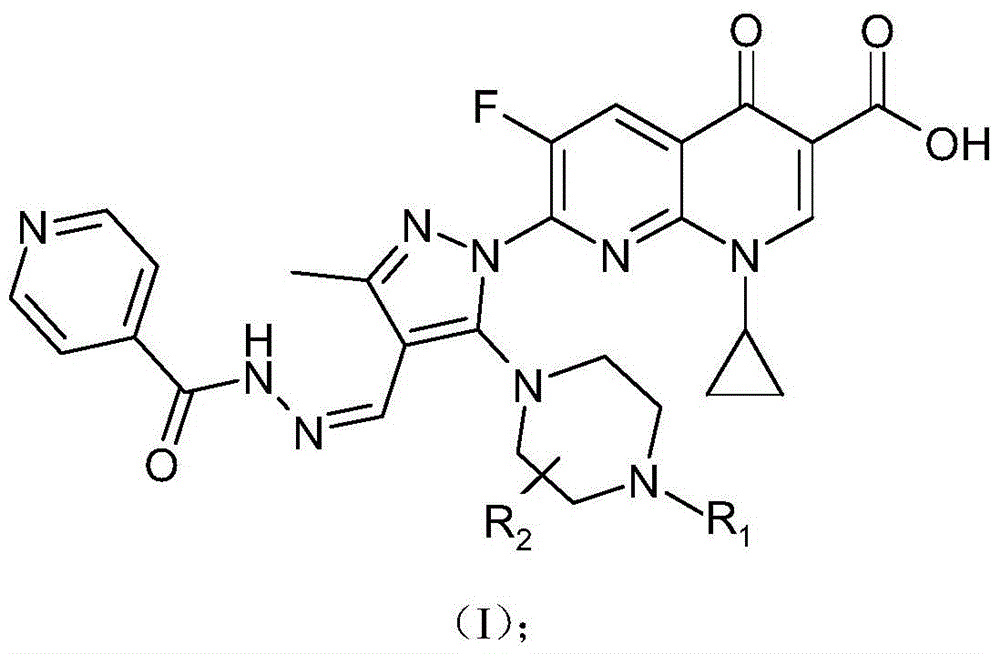
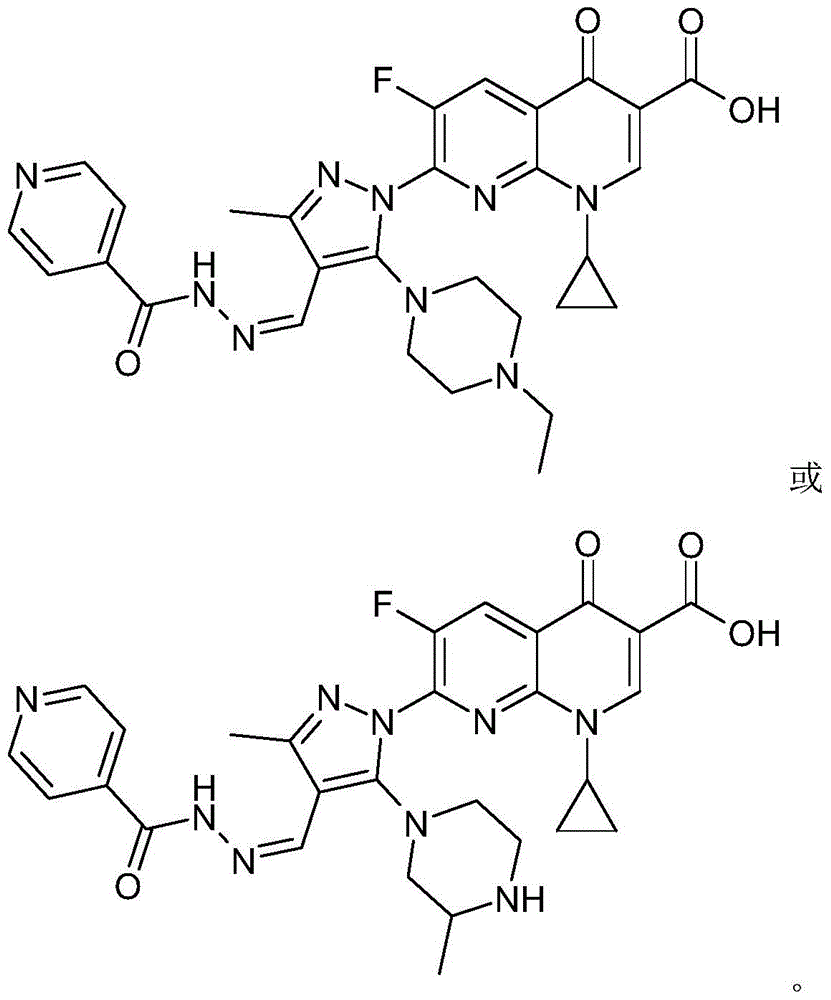
7-(piperazine substituted pyrazole aldehyde isoniazid hydrazone)flunalidone carboxylic acid derivative and its preparation method and application
A technology of flunalidone carboxylic acid and isoniazid, which is applied in the field of preparation of 7-fluoronalidone carboxylic acid derivatives, can solve problems such as low therapeutic index, phototoxicity, and easy drug resistance, and achieve Reduce the probability of drug resistance, reduce toxic and side effects, and increase the effect of anti-tuberculosis activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] The 7-(piperazine-substituted pyrazole aldehyde isoniazid hydrazone) flunalidinone carboxylic acid derivative of this embodiment is 1-cyclopropyl-6-fluoro-7-[3-methyl-4-( Pyridine-4-carbohydrazino)ylidenemethyl-5-piperazin-1-yl-pyrazol-1-yl]-[1,8]naphthyridine-4(1H)-one-3-carboxylic acid, Its chemical structural formula is:
[0050]
[0051] That is, R in formula (I) 1 is a hydrogen atom, R 2 for a hydrogen atom.
[0052] The preparation method of the 7-(piperazine-substituted pyrazole aldehyde isoniazid hydrazone) flunalidinone carboxylic acid derivative of the present embodiment is as follows: take 1.0 g (2.0 mmol) of 1-cyclopropyl-6-fluoro -7-[3-Methyl-4-(pyridine-4-carbohydrazino)ylidenemethyl-pyrazol-1-yl]-[1,8]naphthyridin-4(1H)-one-3- Carboxylic acid (VI) and 0.34g (4.0mmol) of anhydrous piperazine were added to 20ml of anhydrous acetonitrile for reflux reaction for 12h and left overnight; the resulting solid was collected by filtration and recrystallized ...
Embodiment 2
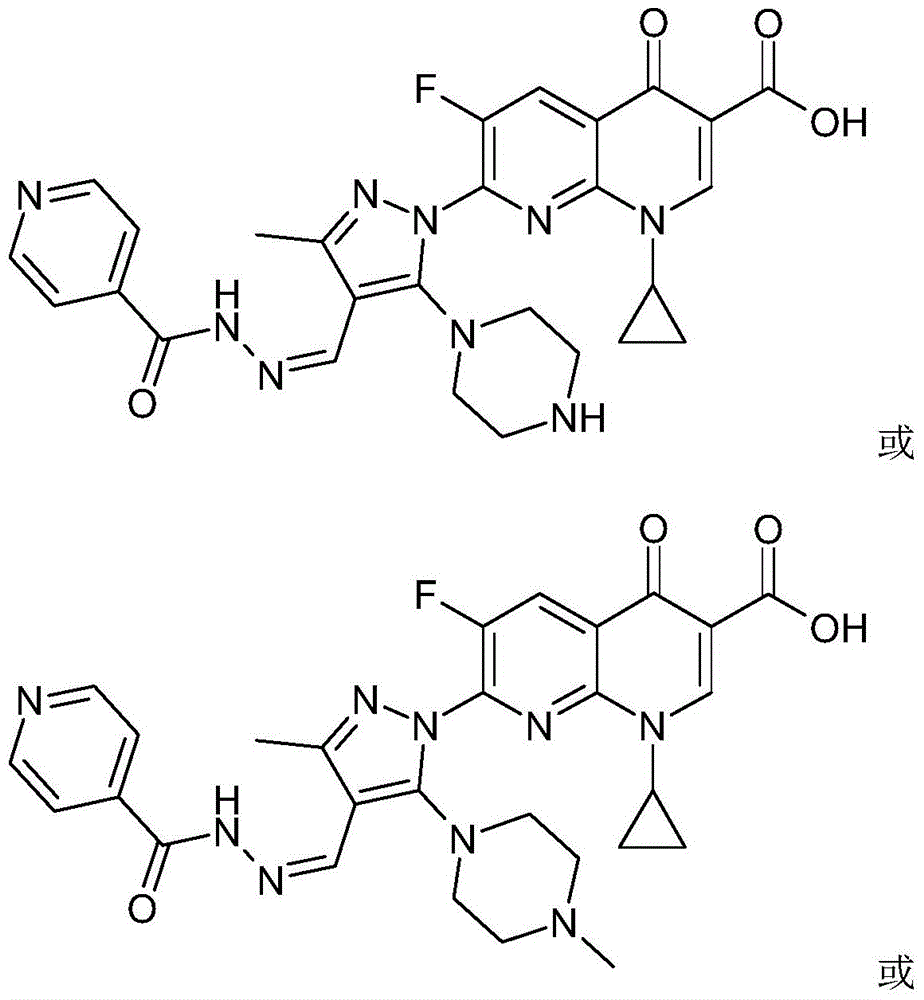
[0054] The 7-(piperazine-substituted pyrazole aldehyde isoniazid hydrazone) flunalidinone carboxylic acid derivative of this embodiment is 1-cyclopropyl-6-fluoro-7-[3-methyl-4-( Pyridine-4-carbohydrazino)ylidenemethyl-5-(4-methylpiperazin-1-yl)-pyrazol-1-yl]-[1,8]naphthyridin-4(1H)-one -3-carboxylic acid, its chemical structural formula is:
[0055]
[0056] That is, R in formula (I) 1 is methyl, R 2 for a hydrogen atom.
[0057] The preparation method of the 7-(piperazine-substituted pyrazole aldehyde isoniazid hydrazone) flunalidinone carboxylic acid derivative of the present embodiment is as follows: take 1.0 g (2.0 mmol) of 1-cyclopropyl-6-fluoro -7-[3-Methyl-5-chloro-4-(pyridine-4-carboxhydrazide)ylidenemethyl-pyrazol-1-yl]-[1,8]naphthyridine-4(1H)- Ketone-3-carboxylic acid (VI) and 0.4g (4.0mmol) of anhydrous methylpiperazine were added to 20ml of anhydrous acetonitrile for reflux reaction for 15h, and left overnight; the resulting solid was collected by filtratio...
Embodiment 3
[0059] The 7-(piperazine-substituted pyrazole aldehyde isoniazid hydrazone) flunalidinone carboxylic acid derivative of this embodiment is 1-cyclopropyl-6-fluoro-7-[3-methyl-4-( Pyridine-4-carbohydrazino)ylidenemethyl-5-(4-ethylpiperazin-1-yl)-pyrazol-1-yl]-[1,8]naphthyridin-4(1H)-one -3-carboxylic acid, its chemical structural formula is:
[0060]
[0061] That is, R in formula (I) 1 is ethyl, R 2 for a hydrogen atom.
[0062] The preparation method of the 7-(piperazine-substituted pyrazole aldehyde isoniazid hydrazone) flunalidinone carboxylic acid derivative of the present embodiment is as follows: take 1.0 g (2.0 mmol) of 1-cyclopropyl-6-fluoro -7-[3-Methyl-5-chloro-4-(pyridine-4-carboxhydrazide)ylidenemethyl-pyrazol-1-yl]-[1,8]naphthyridine-4(1H)- Ketone-3-carboxylic acid (VI) and 0.46g (4.0mmol) of anhydrous ethylpiperazine were added to 20ml of anhydrous acetonitrile for reflux reaction for 24h, and left overnight; the resulting solid was collected by filtration ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com