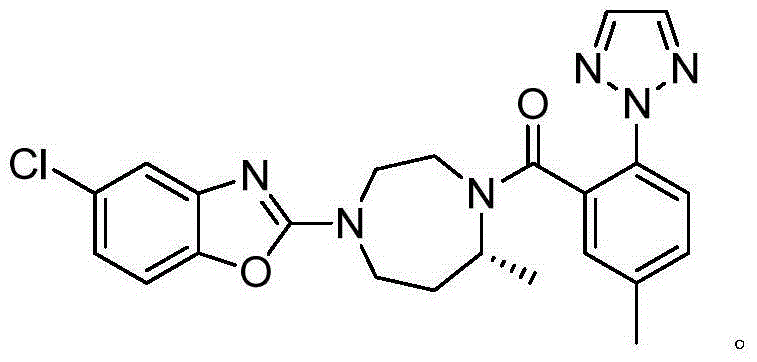
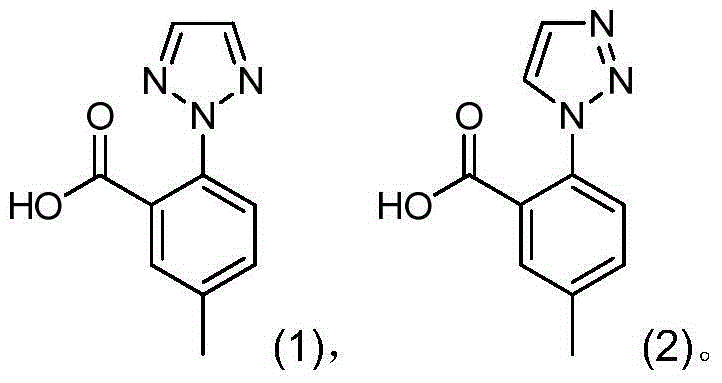
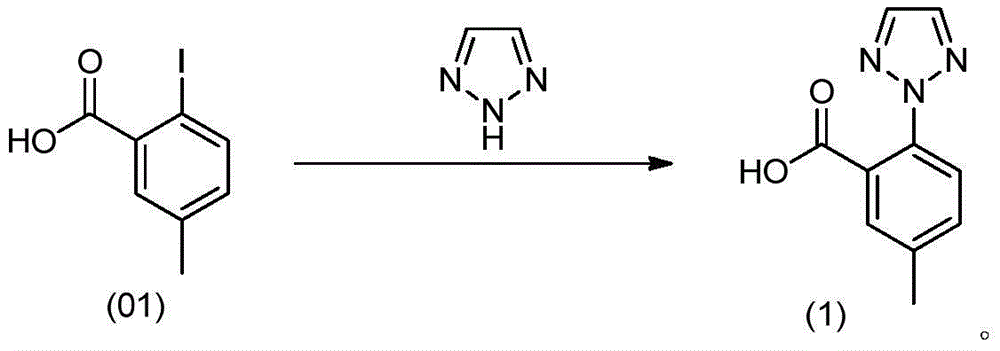
Preparation method of triazoie compound
A compound, triazole technology, applied in the field of preparation of triazole compounds, can solve the problems of complex operation, high cost, low product yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0027] In order to enable those skilled in the art to better understand the technical solutions of the present invention, some non-limiting examples are further disclosed below to further describe the present invention in detail.
[0028] The reagents used in the present invention can be purchased from the market or can be prepared by the methods described in the present invention.
[0029] In the present invention, g means gram, mL means milliliter, rpm means revolution / minute, and mol / L means mole / liter.
Embodiment 1
[0031] In the reactor, add 250mL of acetone, stir, add 26.2g of compound (01), then add 35.0g of potassium carbonate, 0.38g of cuprous iodide (CuI), 7.6g of 1,2,3-triazole and 200mL of acetone. The external temperature was raised to 70° C., during which a large amount of gas was generated, and the reaction solution was refluxed for 5 hours. Then the reaction solution was distilled under reduced pressure. When the reaction system was relatively viscous, 30 mL of water was added, and the distillation was continued until there was no acetone in the distillate (gas phase detection without acetone). 300 mL of water was added to the residue after distillation, and 6 mol / L hydrochloric acid was added dropwise at room temperature to adjust the pH of the system to 1-2 to obtain a khaki suspension. Stir for 15 minutes, filter, and wash the solid with water 3 times, 50 mL each time. The obtained solid was vacuum-dried to dryness at 70°C to obtain 19.45 g of a light green solid, which wa...
Embodiment 2
[0033] In the reactor, add 350mL butanone, stir, add 26.2g of compound (01), then add 44.15g of potassium carbonate trihydrate, 0.38g of cuprous iodide (CuI), and 7.6g of 1,2,3-triazole. The external temperature was raised to 85° C., during which a large amount of gas was generated, and the reaction solution was refluxed for 5 hours. Then the reaction solution was distilled under reduced pressure at 40°C. When the reaction system was relatively viscous, 50 mL of water was added, and the distillation was continued until the distillate was free of butanone (gas phase detection without butanone). 300 mL of water was added to the residue after distillation, and concentrated hydrochloric acid was added dropwise at room temperature to adjust the pH of the system to 1-2 to obtain a yellow suspension. Stir for 30 minutes, filter, and wash the solid with water 3 times, 60 mL each time. The obtained solid was vacuum-dried to dryness at 60° C. to obtain 19.29 g of a light green solid, w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com