Synthesis technology for capecitabine
A synthetic process, capecitabine technology, applied in the field of chemical drug synthesis, can solve problems such as existing risks and high costs, and achieve the effect of improving quality, reducing quantity and limit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
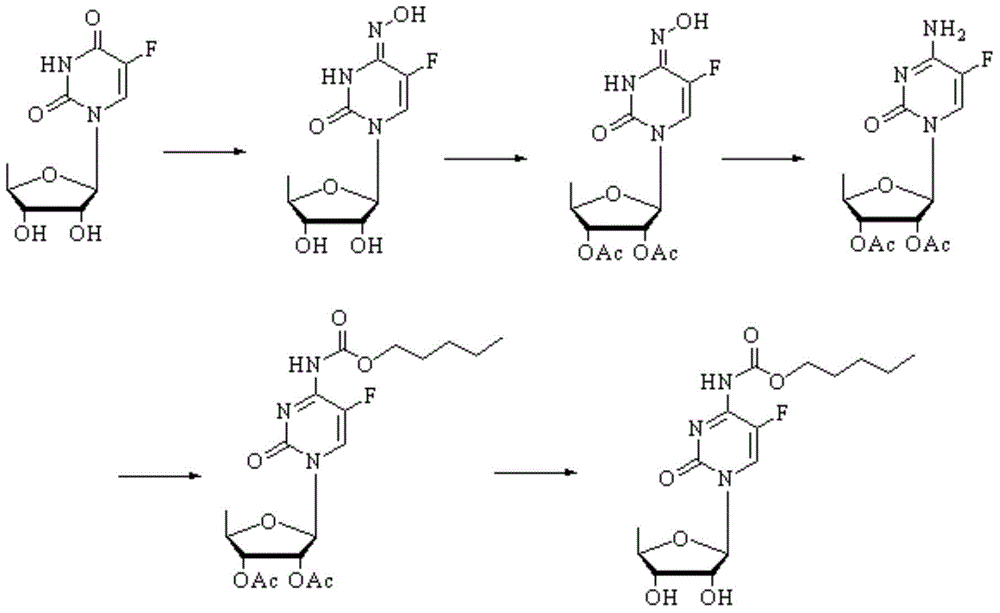
[0018] Add 5′-deoxy-5-fluorouridine (40.7mmol) and hydroxylamine hydrochloride (40.7mmol) to methanol (100mL), add triethylamine (5mL) under stirring, react at 50-60°C for 2h, cool and filter , and washed to obtain a white solid (10 g, yield 94.4%). Dichloromethane (100mL) was added to 5'-deoxy-ribose-5-fluoro-4-hydroxylamine-3,4 dihydropyrimidine (38.3mmol) and potassium carbonate (38.3mmol), and acetic anhydride (91.9 mmol), react at 10-15°C for 5h, concentrate to dryness, wash the residue with water, filter and dry to obtain a white solid (12.9g, yield 97.7%). Add 2',3'-di-O-acetyl-5'-deoxy-ribose-5-fluoro-4-hydroxylamino-3,4 dihydropyrimidine (28.9mmol) and hydrochloric acid (10ml) to water (50mL) Add zinc chloride (57.8mmol) under stirring, react at 70-80°C for 3.5h, wash with water after cooling, filter, and dry to obtain a white solid (8.6g, yield 90.5%). Under ice-cooling, slowly drop n-pentyl chloroformate (21.5mmol) into N,N-diisopropylethylamine (0.46mL) and 2',3'...
Embodiment 2
[0020] Add 5′-deoxy-5-fluorouridine (40.7mmol) and hydroxylamine hydrochloride (40.7mmol) to ethanol (100mL), add triethylamine (5mL) under stirring, react at 70-80°C for 2h, cool and filter , and washed to obtain a white solid (9.6 g, yield 90.6%). Add 5'-deoxy-ribose-5-fluoro-4-hydroxylamino-3,4 dihydropyrimidine (38.3mmol) and triethylamine (38.3mmol) to acetic anhydride (100mL), add acetic anhydride (91.9mmol) under stirring mmol), reacted at 25-30°C for 5h, cooled and concentrated to dryness, the residue was washed with water, filtered and dried to obtain a white solid (12.3g, yield 92.8%). Add 2',3'-di-O-acetyl-5'-deoxy-ribose-5-fluoro-4-hydroxylamino-3,4-dihydropyrimidine (27.7mmol) and hydrochloric acid (10ml) to methanol (50mL) Add zinc chloride (54.9mmol) under stirring, react at 40-50°C for 3.5h, wash with water after cooling, filter, and dry to obtain a white solid (8.2g, yield 86.0%). Under ice-cooling, n-pentyl chloroformate (20.4mmol) was slowly dropped into N...
Embodiment 3
[0022]Add 5′-deoxy-5-fluorouridine (40.7mmol) and hydroxylamine hydrochloride (40.7mmol) to 50% ethanol solution (100mL), add triethylamine (5mL) under stirring, react at 65-70°C for 2h, After cooling, it was filtered and washed to obtain a white solid (9.4 g, yield 88.7%). Dichloromethane (100mL) was added to 5'-deoxy-ribose-5-fluoro-4-hydroxylamine-3,4 dihydropyrimidine (36.1mmol) and triethylamine (36.1mmol), and acetic anhydride ( 86.3 mmol), reacted at 0-5°C for 5 h, cooled and concentrated to dryness, the residue was washed with water, filtered and dried to obtain a white solid (12.1 g, yield 91.8%). Add 2',3'-di-O-acetyl-5'-deoxy-ribose-5-fluoro-4-hydroxylamine-3,4-dihydropyrimidine (27.2mmol) and hydrochloric acid (10ml) to ethanol (50mL) Add zinc chloride (57.8mmol) under stirring, react at 50-55°C for 3.5h, wash with water after cooling, filter, and dry to obtain a white solid (8.1g, yield 85.1%). Under ice-cooling, n-pentyl chloroformate (20.2 mmol) was slowly dro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com