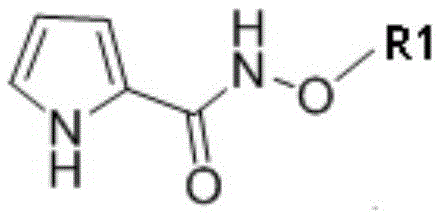
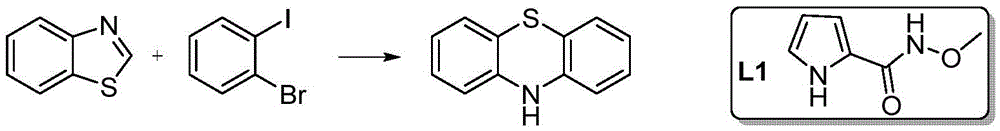
A method for synthesizing phenothiazine compounds
A technology of phenothiazine compounds, which is applied in the field of synthesizing phenothiazine compounds, can solve the problems of synthesis of phenothiazine compounds that have not been reported, and achieve the effects of low solvent toxicity, low reaction temperature, and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: the synthesis of phenothiazine
[0025]
[0026] 135mg (1mmol) benzothiazole, 566mg (2mmol) o-bromoiodobenzene, 25mg (0.1mmol) CuSO 4 ·5H 2 O, 14 mg (0.1 mmol) Ligand L1, 138 mg (1 mmol) K 2 CO 3 , 2g PEG-400, added to a 10mL reaction tube, sealed, and reacted at 50°C for 20h. After the reaction stopped, it was extracted with ethyl acetate, washed with water, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and the filtrate was distilled under reduced pressure, separated and purified by silica gel column chromatography to obtain 160 mg of phenothiazine with a yield of 80%.
[0027] MS (EI + ):m / z:199(M + ,100),167(M-S,65); 1 HNMR (400MHz, DMSO-d 6 )δ8.61(s,1H,NH),6.99(td,J=7.6,1.4Hz,2H,ArH),6.94–6.88(m,2H,ArH),6.78–6.69(m,4H,ArH).
Embodiment 2
[0028] Embodiment 2: the synthesis of 2-chlorophenothiazine
[0029]
[0030]135 mg (1 mmol) benzothiazole, 158 mg (0.5 mmol) 2-bromo-4-chloro-1-iodobenzene, 16 mg (0.2 mmol) CuO, 2 mg (0.01 mmol) ligand L2, 28 mg (0.5 mmol) KOH, Add 2g of PEG-300 into a 10mL reaction tube, seal it, and react at 90°C for 3h. After the reaction stopped, extracted with ethyl acetate, washed with water, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, the filtrate was distilled under reduced pressure, separated and purified by silica gel column chromatography to obtain 151 mg of 2-chlorophenothiazine, the yield 65%.
[0031] MS (EI + ):m / z:198(M-Cl,100),233(M + ,78),235(M + ,25),201(M-S,25). 1 HNMR (400MHz, DMSO-d 6 )δ8.77(brs,1H,NH),7.04–6.98(m,1H,ArH),6.96–6.88(m,2H,ArH),6.82–6.75(m,2H,ArH),6.73–6.66(m ,2H,ArH).
Embodiment 3
[0032] Embodiment 3: the synthesis of 2-fluorophenothiazine
[0033]
[0034] 135 mg (1 mmol) benzothiazole, 300 mg (1.0 mmol) 2-bromo-4-fluoro-1-iodobenzene, 6.4 mg (0.1 mmol) Cu, 21.6 mg (0.1 mmol) ligand L2, 978 mg (3 mmol) Cs 2 CO 3 , 2g PEG-100, put into a 10mL reaction tube, seal it, and react at 110°C for 14h. After the reaction stopped, extracted with ethyl acetate, washed with water, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, the filtrate was distilled under reduced pressure, separated and purified by silica gel column chromatography to obtain 156 mg of 2-fluorophenothiazine, the yield 72%.
[0035] MS (EI + ):m / z:217(M + ,100),185(M-S,88). 1 HNMR (400MHz, DMSO-d 6 )δ8.81(s,1H,NH),7.01(td,J=7.7,1.5Hz,1H,ArH),6.97–6.89(m,2H,ArH),6.78(t,J=7.5Hz,1H, ArH), 6.70(d, J=7.9Hz, 1H, ArH), 6.59(td, J=8.5, 2.7Hz, 1H, ArH), 6.53(dd, J=10.5, 2.6Hz, 1H, ArH).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com