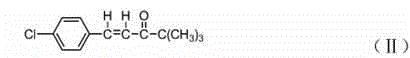Synthetic method for bactericide tebuconazole
A synthetic method, the technology of tebuconazole, which is applied in the field of synthesis of the fungicide tebuconazole, can solve the problems of isomers and low product yield, and achieve the effect of no by-products and mild reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Synthesis of 4,4-dimethyl-1-(4-chlorophenyl)-1-penten-3-one:
[0031] Add sodium methoxide, 2mol p-chlorobenzaldehyde, 1mol pinaconone and 1mol potassium bicarbonate with a mass concentration of 55% to the reaction flask, stir and heat up to 55°C, reflux at this temperature for 2h; heat up to 70°C and keep for 1.5h, Then cool to room temperature and filter to obtain 4,4-dimethyl-1-(4-chlorophenyl)-1-penten-3-one; the yield is 98.9%, and the purity is 99.4%;
Embodiment 2
[0033] Identical with embodiment 1 operating steps, difference is that weakly basic catalyst is sodium bicarbonate; Productive rate is 99.4%, and purity is 99.5%;
Embodiment 3
[0035] Synthesis of 4,4-Dimethyl-1-(4-chlorophenyl)-3-pentanone
[0036] 4,4-dimethyl-1-(4-chlorophenyl)-1-penten-3-one 4mol obtained in Example 2 was put into a hydrogenation kettle, the solvent methanol was added, and then 2mol of platinum oxide and Lu Hong 1 mol of the solution; replaced with nitrogen three times and hydrogen three times successively, then heated up to 65°C, and at this temperature, hydrogen was introduced at a pressure of 3.0 MPa until the reaction stopped absorbing hydrogen, and the reaction solution was lowered to normal temperature and normal temperature. pressure, and the filtrate was distilled under reduced pressure to remove the solvent to obtain 4,4-dimethyl-1-(4-chlorophenyl)-3-pentanone; the yield was 99.5%, and the purity was 99.3%;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com