Urapidil oxidation product and preparation method thereof
An oxidation product, urapidil technology, applied in the direction of organic chemistry, can solve the problem of the degradation product not being identified, and achieve the effect of improving the quality standard
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Preparation and isolation of compound I
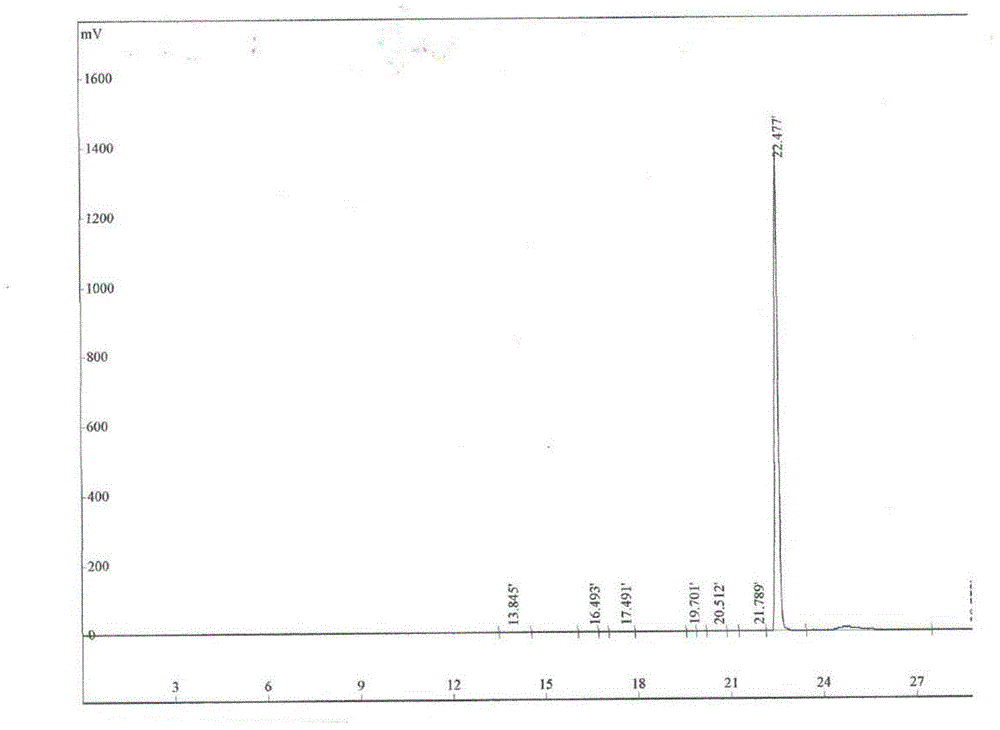
[0037] In the there-necked flask, add the urapidil raw material (2g, 5.0mmol) and 20ml of dimethylformamide and heat to 70°C. After the raw material is completely dissolved, add 15% hydrogen peroxide (100ml) dropwise, and react at 70°C for 5 hours and stop heating. , the reaction solution was added to a saturated sodium sulfite solution (100ml) to quench the reaction, the reaction solution was concentrated and passed through a preparative column to obtain the target product, which was subjected to preparative chromatography.
[0038] Preparative Chromatography: Separation of oxidative degradation products was performed on reversed-phase chromatography. In order to obtain pure compounds, separations were carried out using chromatographic separations with different mobile phases.
[0039] The separation of urapidil oxidation products was performed on a preparative HPLC chromatogram equipped with an Innoval ODS-DAC column (250*250...
Embodiment 2
[0052] In the there-necked flask, add urapidil raw material (1g, 2.5mmol) and 40 milliliters of methanol and heat to 50°C. After the raw materials are completely dissolved, add 20% hydrogen peroxide (8ml) dropwise, and react at 50°C for 4 hours to stop heating. The reaction was quenched by adding saturated sodium sulfite solution (10 ml), and the reaction solution was concentrated and passed through a preparative column to obtain the target product.
[0053] Adopt the method of embodiment 1 to carry out preparation and separation.
Embodiment 3
[0055]In the there-necked flask, add urapidil raw material (2g, 5.0mmol) and 20 milliliters of toluene and heat to 60°C, after the raw material is completely dissolved, add 30% hydrogen peroxide (10ml) dropwise, and the reaction solution is reacted at 60°C for 6 hours to stop heating, and the reaction solution The reaction was quenched by adding saturated sodium sulfite solution (10 ml), and the reaction solution was concentrated and passed through a preparative column to obtain the target product.
[0056] Adopt the method of embodiment 1 to carry out preparation and separation.
[0057] Structural illustration of compound I:
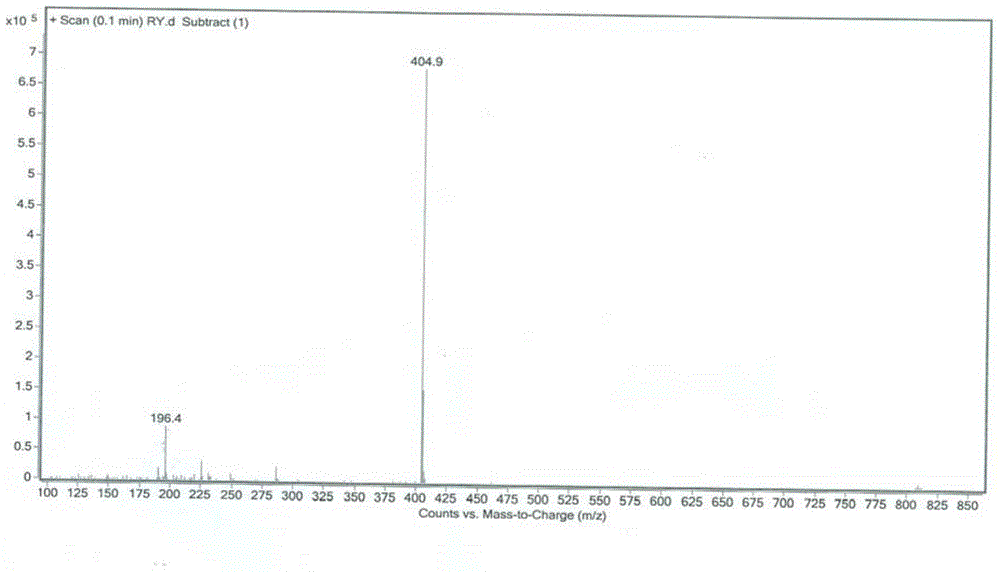
[0058] Mass spectrometry:
[0059] Conditions: Linear ion trap electrostatic field orbitrap combined mass spectrometer LTQ Orbitrap XL mass spectrometer to obtain high-resolution mass spectra. Electrospray ionization was used.
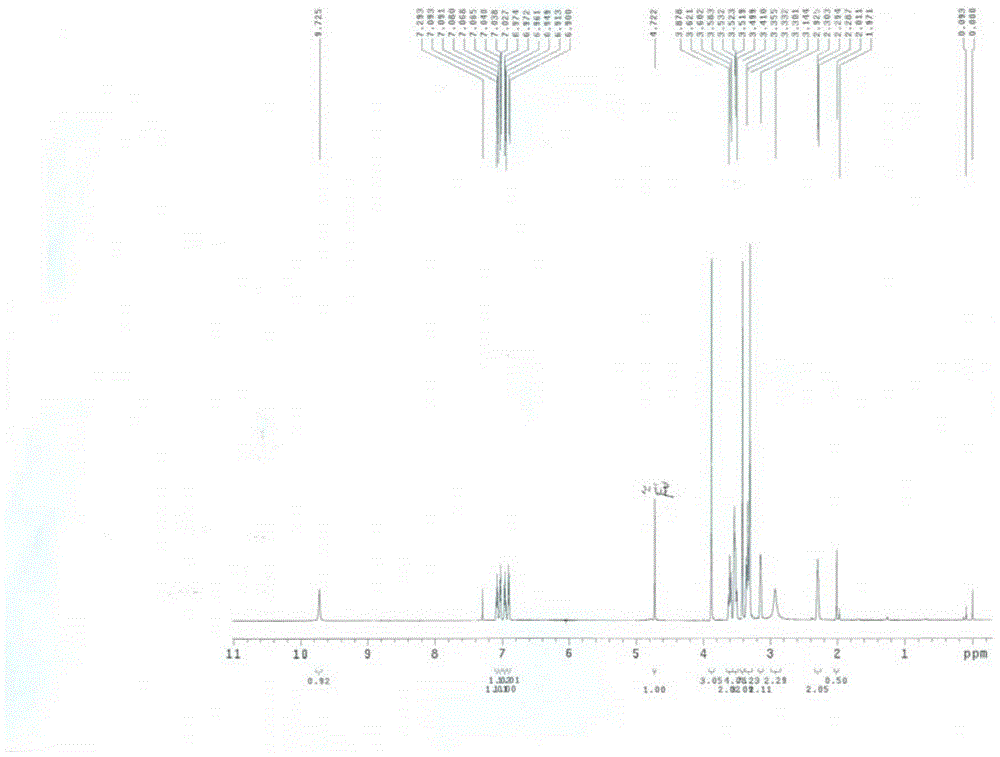
[0060] NMR spectroscopy:
[0061] Conditions: H and C were performed on a BRUKER AVANCE 600 NMR spectrometer.
[0062] s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com