Synthetic method for p-hydroxyphenyl glycine
The technology of L-para-hydroxyphenylglycine and para-hydroxyphenylglycine, which is applied in the field of medicine, can solve the problems of low production capacity, high product light absorption value, large amount of mother liquor eliminated, etc., and achieves reduction of waste water generation, simple synthesis process and separation. easy-to-obtain effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] The invention provides a kind of synthetic method of L-p-hydroxyphenylglycine, comprising the following steps:
[0026] A) mixed reaction of DSG and resolving agent to obtain the double salt formed by L-p-hydroxyphenylglycine and resolving agent;
[0027] B) dissolving the double salt formed by L-p-hydroxyphenylglycine and a resolving agent in water, mixing with lye, stirring, crystallizing and refining to obtain L-p-hydroxyphenylglycine;
[0028] The resolving agent is D-mandelic acid or D-phenylethanesulfonic acid.
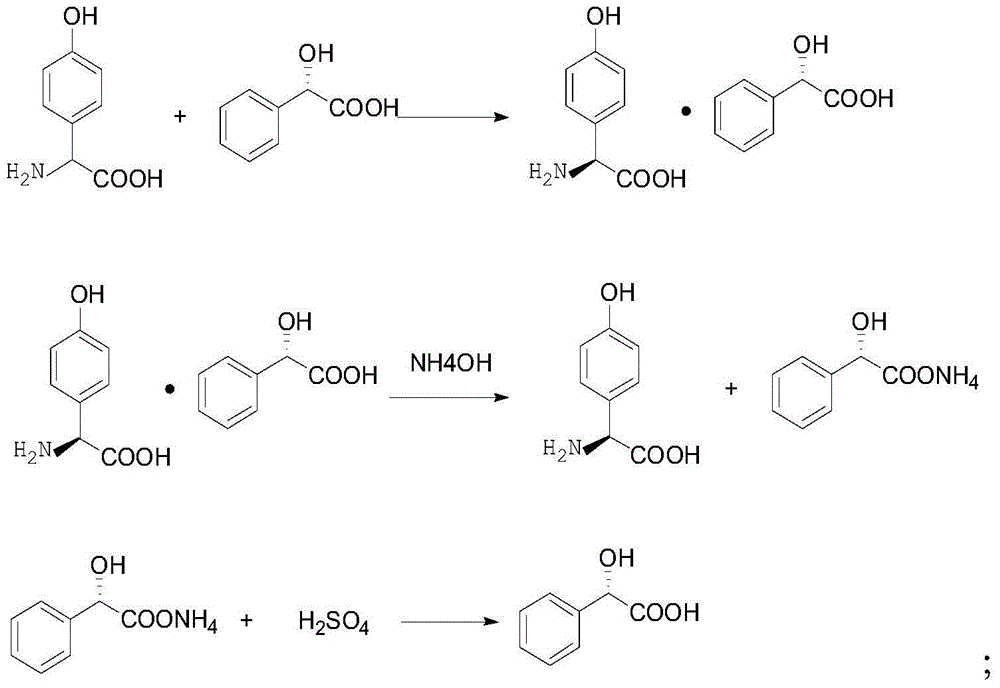
[0029] When described resolving agent is D-mandelic acid, its reaction process is:
[0030]
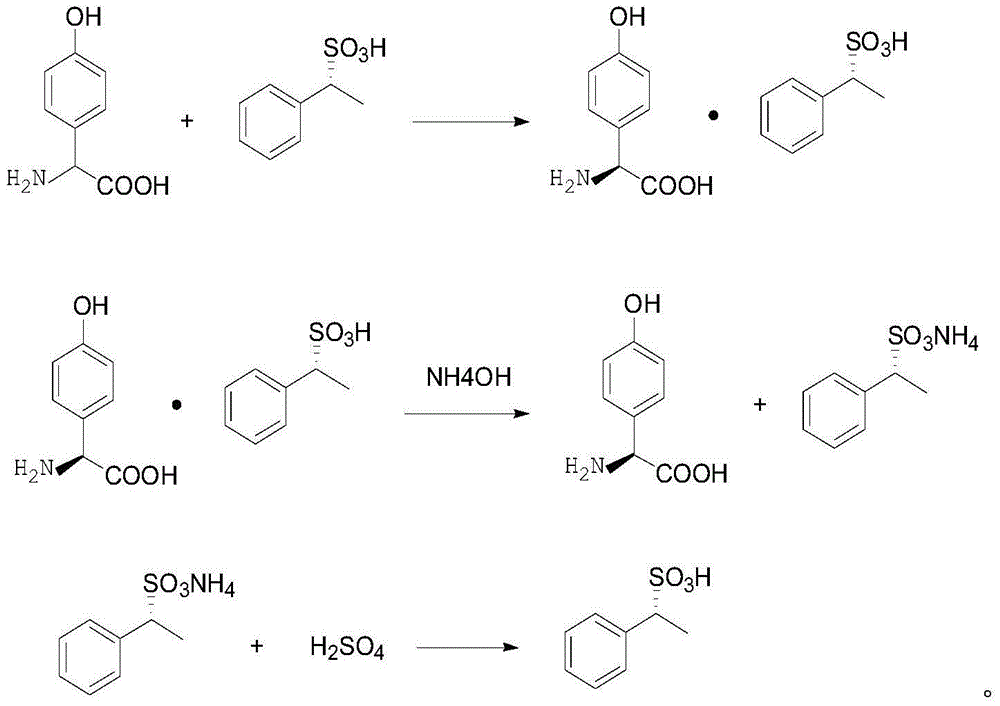
[0031] When described resolving agent is D-phenylethanesulfonic acid, reaction process is:
[0032]
[0033] In the present invention, firstly, the mixed reaction of DSG and the resolving agent is carried out to obtain the double salt formed by the L-p-hydroxyphenylglycine and the resolving agent. The present invention does not have special limitation to...
Embodiment 1
[0053] (1) Hydrolysis and refining of L-p-hydroxyphenylglycine
[0054] Get 500g (1.566mol) of the double salt formed by L-p-hydroxyphenylglycine and the resolution agent D-mandelic acid, add in 2500g of pure water, slowly add dropwise a concentration of 18% ammonia water, neutralize to a pH value of 4, and suction filter to obtain The crude product of L-p-hydroxyphenylglycine, the obtained crude product of L-p-hydroxyphenylglycine was put into 300g of pure water, 78.3g (0.783mol) of 98% concentrated sulfuric acid was added, heated to 85°C to dissolve, 0.5g of activated carbon was added, kept for 1h, filtered, Add 18% ammonia water dropwise to the filtrate until the pH value is 4, continue to stir for 0.5h, filter and dry to obtain 240g of the product, the yield is 91.7%, the specific optical rotation of the product is -158.3°, the content is 99.45%, and the alkali absorbance is 0.024. Glycine was not detected. The obtained solution is a solution containing a resolving agent ...
Embodiment 2
[0060] (1) Hydrolysis and refining of L-p-hydroxyphenylglycine
[0061] Take 500 g (1.566 mol) of the double salt formed by L-p-hydroxyphenylglycine and the resolution agent D-mandelic acid, add it to 1500 g of pure water, slowly add 30% sodium hydroxide dropwise, neutralize to pH 6, and filter with suction , to obtain the crude product of L-p-hydroxyphenylglycine, put the crude product of L-p-hydroxyphenylglycine into 300g pure water, add 184.38g (1.566mol) of 31% concentrated hydrochloric acid, heat to 85°C to dissolve it, add 0.5g of activated carbon, and keep it warm for 1h. Filtrate, add 30% sodium hydroxide dropwise to the filtrate until the pH value is 6, continue to stir for 0.5h, suction filter and dry to obtain 243g of the product, the yield is 92.8%, the specific optical rotation of the product is -158.1°, the content is 99.52%, the alkali absorbance is 0.020, the right Spin-p-hydroxyphenylglycine was not detected. The obtained solution is a solution containing a r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com