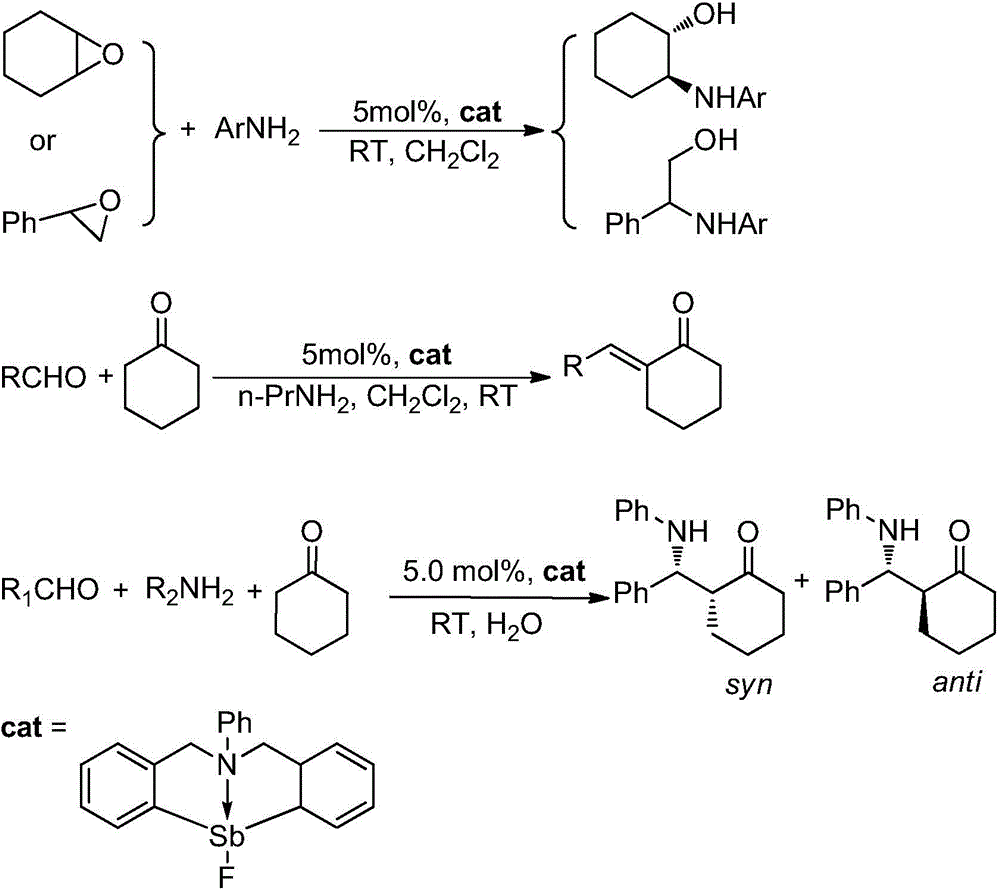
Catalytic synthesis application based on frustrated Lewis acid-base pair
A Lewis acid-base pair, catalytic reaction technology, applied in the field of synthesis of β-amino ketones, can solve the problems of difficult to catalyze organic reactions, difficult to reuse, air-sensitive, etc., and achieves low cost, easy operation, and simple reaction conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0023] Add 0.429 g C into a 50 mL two-necked flask 6 h 5 N(C 6 h 4 CH 2 ) 2 SbCl (1.0 mmol), dissolved in 15mL THF solution, and then under nitrogen protection, will contain 0.195g AgBF 4 (1.0 mmol) tetrahydrofuran solution was transferred into the antimony compound solution, stirred at room temperature in the dark for 4 h, then stopped the reaction, and filtered. Add 1.0 mL of n-hexane to the filtrate and keep it at room temperature for 24 h to obtain colorless crystals, which are the target compound hindered Lewis acid-base pair PhN(CH 2 C 6 h 4 ) 2 SbF, the yield is 95.0%.
preparation example 2
[0025] Add 0.05mmol hindered Lewis acid-base pair PhN(CH 2 C 6 h 4 ) 2 SbF, 2.0 mL of dichloromethane, 1.0 mmol of 1,2-epoxycyclohexane and 1.0 mmol of aniline (Ar=Ph) were placed in a water bath reactor with magnetic stirring, and reacted at 25° C. for 8 hours. TLC followed the reaction until the reaction was complete. The reaction result is: (E)-2-anilinocyclohexanol, the yield is 90%, and the selectivity of (E)-2-anilinocyclohexanol is 100%.
preparation example 3
[0027] Add 0.05mmol hindered Lewis acid-base pair PhN(CH 2 C 6 h 4 ) 2 SbF and 2.0mL dichloromethane, 1.0mmol 1,2-epoxycyclohexane and 1.0mmol o-methylaniline (Ar=o-CH 3 C 6 h 4 ), placed in a water-bath reactor with magnetic stirring, and reacted for 10 hours at 25°C. TLC followed the reaction until the reaction was complete. The reaction result is: (E)-2-[(2-methylphenyl)amino]-cyclohexanol, the yield is 82%, (E)-2-[(2-methylphenyl)amino]-cyclohexanol Selectivity is 100%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com