1,1',1''-trishydroxy triptycene and synthesis method thereof
A technique for the synthesis of trihydroxytriptytylene, which is applied to the preparation of amino compounds, chemical instruments and methods, and compounds of Group 4/14 elements of the periodic table, etc. It can solve the problem that the functionalization of triptylene skeleton is rarely realized, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
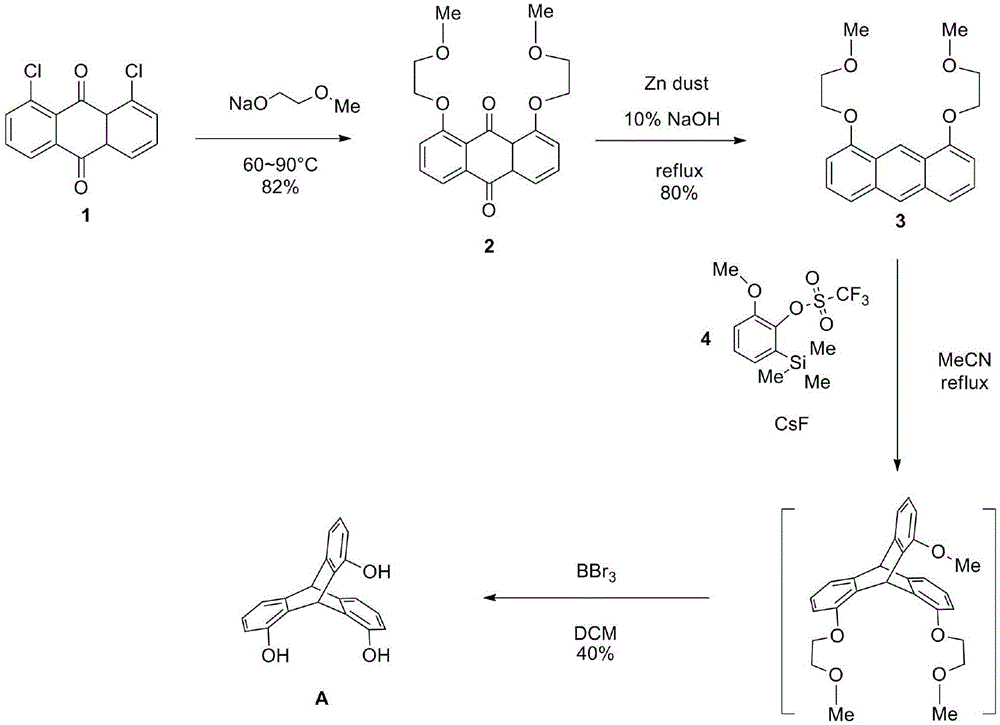
[0007] The present invention will be further described below in conjunction with specific methods.
[0008] 1. Synthesis of 1,8-dialkoxyanthraquinone 2: At 0°C, metal sodium (7.47g, 324mmol, 3.0equiv.) was added in batches to hexanediol monomethyl ether (170.76mL, 2163mmol, 20.0 equiv.), after the metallic sodium disappeared, 1,8-dichloroanthraquinone (30.0 g, 108 mmol, 1.0 equiv.) was added to the above solution, and the reaction temperature was raised to 60° C. and stirred for 8 h.
[0009] Post-processing: After the reaction was completed, the reaction temperature was slowly lowered to 0° C., water (100 mL) was added and stirred for about 10 min, and then DCM (100 mL) was added to separate the reactants. The organic phase was taken with a separatory funnel, and the aqueous phase was extracted with DCM (200 mL×3). After the organic phases were combined, they were washed with saturated NaCl solution (200 mL×1), followed by anhydrous NaCl 2 SO 4 Dry and filter. The obtaine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com