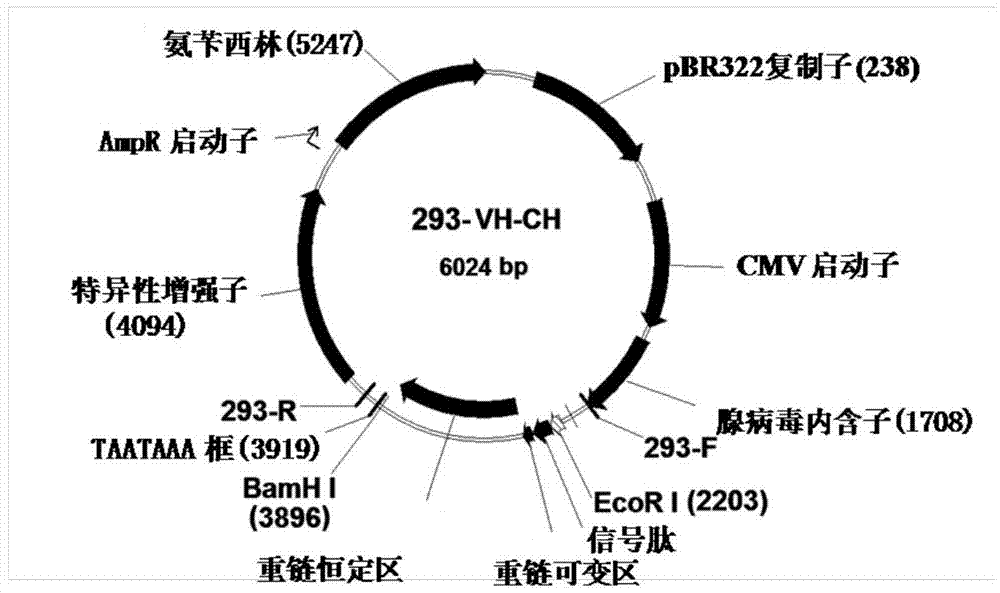
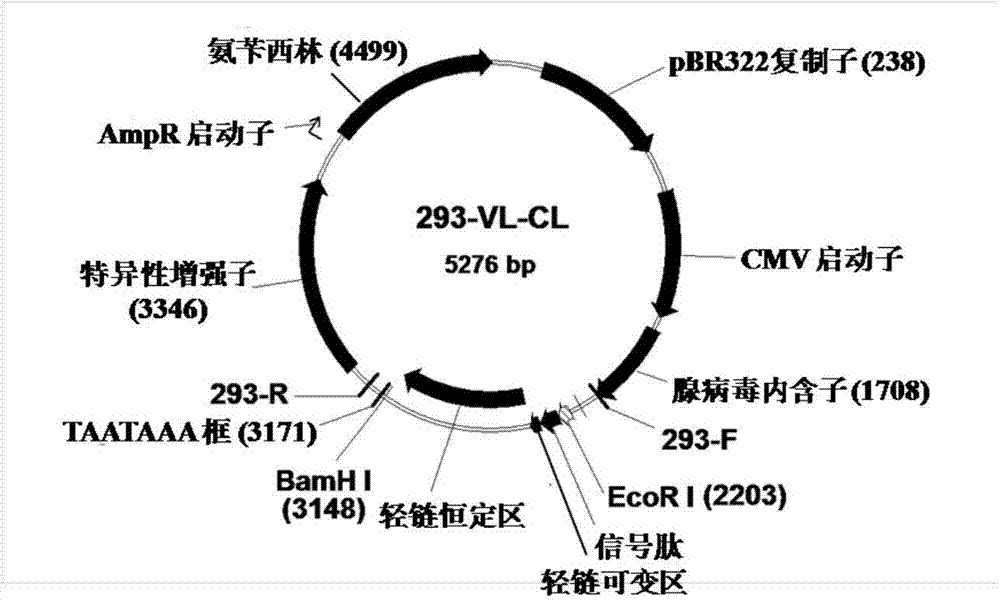
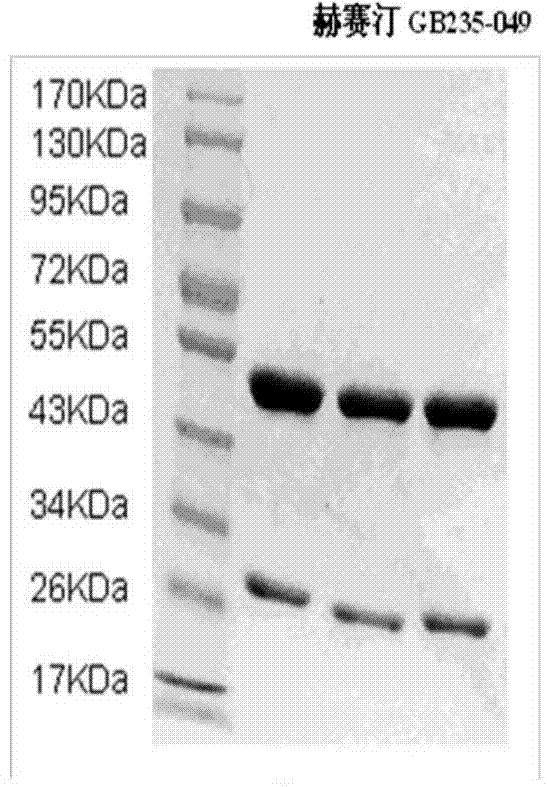
Fully humanized HER2 antibody as well as encoding gene and application of antibody
A fully human, antibody-based technology, applied in applications, antibodies, genetic engineering, etc., can solve problems such as the incomplete understanding of the drug resistance mechanism of Herceptin, the loss of phosphorylated PTEN, and abnormal signal transmission pathways
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 Screening positive clones of scFv from a fully human scFv phage library
[0042] Human HER2 (extracellular domain)-Fc fusion protein (hereinafter referred to as hHER2-Fc) antigen (purchased from Sino Biological Company, product number: 10004-H02H) was washed with phosphate buffered saline PBS (0.01M Na 2 HPO 4 12H 2 O+0.002MKH 2 PO 4 +0.14M NaCl+0.002M KCl, pH=8.6) was diluted to 5 μg / ml, added to a microtiter plate at 100 μl / well, and coated overnight at 4°C. After the plate was washed 4 times with PBST (PBS buffer containing 0.05% Tween 20), 300 μl / well of 5% BSA (bovine serum albumin, product number: A7030, purchased from Sigma) was added, and blocked at 37° C. for 1 hour. Then wash the plate twice with PBST. will contain 7×10 10An independently cloned fully human scFv phage antibody library (this antibody library was constructed by Eureka (Beijing) Biotechnology Co., Ltd. by combining multiple antibody variable region genes of healthy human lymphocyt...
Embodiment 2
[0043] Example 2 Enzyme-linked immunosorbent assay (ELISA) identification of scFv phage-positive clones
[0044] The hHER2-Fc antigen was diluted to 2 μg / ml with PBS (pH=8.6), added to the microtiter plate at 100 μl / well, and coated overnight at 4°C. After washing the plate 4 times with PBST, add 300 μl / well of 5% BSA, and block for 1 hour at 37°C. Then wash the plate twice with PBST, add 100 μl / well phage-positive clone suspension, and incubate at 37° C. for 2 hours. Wash the plate 4 times with PBST, add HRP (horseradish peroxidase)-labeled anti-M13 phage antibody (purchased from GE, article number: 27-9421-01, diluted with PBST 1:5000, 100 μl / well), and incubate at room temperature for 1 Hour. Wash the plate 4 times with PBST, add 100 μl / well color developing solution (soluble one-component TMB substrate solution, purchased from Tiangen Company, article number: PA107-01), incubate at room temperature for 15 minutes to develop color, add 50 μl / well stop solution (1M sulfur...
Embodiment 3
[0048] Example 3 ELISA method detected 102 scFv phage positive clones and monkey HER2-Fc (hereinafter referred to as mkHER2-Fc, purchased from Sino Biological Company, article number: 90295-C02H), mouse HER2-Fc (hereinafter referred to as moHER2-Fc , purchased from Sino Biological Company, product number: 50714-M02H), human HER1-Fc (hereinafter referred to as hHER1-Fc, purchased from Sino Biological Company, product number: 10001-H02H), human HER3-Fc (hereinafter referred to as hHER3-Fc , purchased from Sino Biological Company, product number: 10201-H05H) and human HER4-Fc (hereinafter referred to as hHER4-Fc, purchased from Sino Biological Company, product number: 10363-H02H) antigen cross-reaction.
[0049] The method is the same as in Example 2, only the coated hHER2-Fc antigens are replaced with mkHER2-Fc, moHER2-Fc, hHER1-Fc, hHER3-Fc and hHER4-Fc antigens respectively.
[0050] The results showed that 96 positive clones of scFv phage had cross-reaction with mkHER2-Fc ant...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com