Metal organic framework adsorbent as well as preparation method and application thereof
A metal-organic framework and metal-organic framework technology are applied in the field of metal-organic framework adsorbents and their preparation, which can solve the problem of low selectivity, and achieve the effects of large adsorption capacity, high-efficiency separation selectivity, and strong adsorption effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
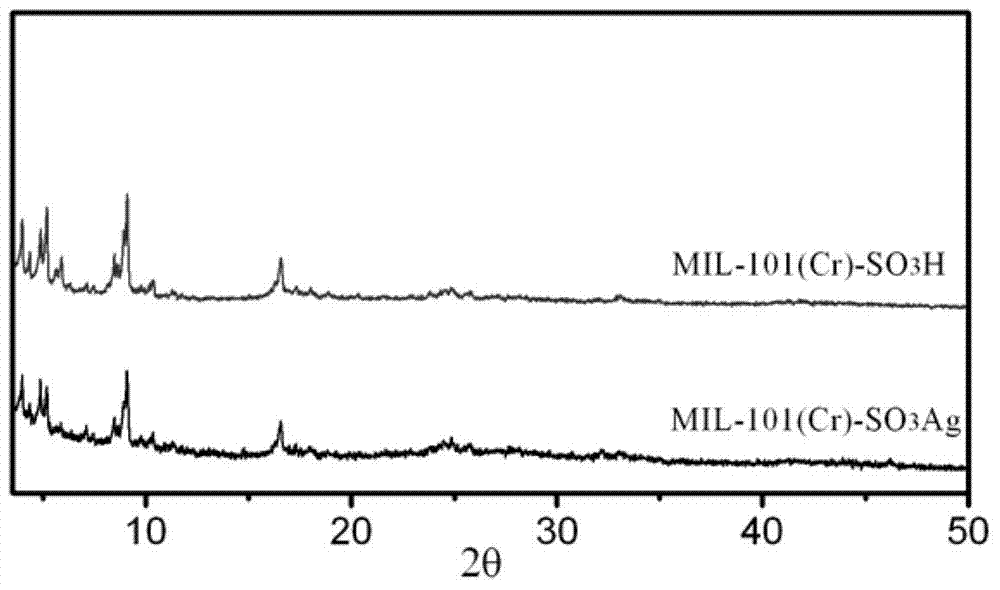
[0041] (1) MIL-101(Cr)-SO 3 Preparation of H: 4g chromium nitrate nonahydrate, 5.4g sodium monosulfonate terephthalic acid, 60g deionized water and 0.6g hydrofluoric acid (~40wt%) (ratio: 1mmol: 2mmol: 6g: 0.06g) Add it into the reaction kettle, stir it evenly with ultrasonic waves, and then react at 190°C for 24 hours to obtain ((Cr))-MIL-101-SO 3 H. The initially synthesized MOF was washed with deionized water and ethanol for 2 to 3 times respectively, centrifuged, filtered, and finally vacuum-dried at 120°C for 12 hours to obtain the purified MIL-101(Cr)-SO 3 H, the specific surface area is 1856m 2 / g, the pore volume is 1.35cm 3 / g. Unless otherwise specified, the following examples are MIL-101(Cr)-SO synthesized by this method 3 H.
[0042] (2) Adsorbent MIL-101(Cr)-SO 3 The preparation of Ag: get the purified MIL-101(Cr)-SO in 250mg step (1) 3 H was added to the container. Then add 20ml of AgBF at a concentration of 40mg / ml 4 In the solution of acetonitrile / wat...
Embodiment 2
[0045] Adsorbent MIL-101(Cr)-SO 3 The preparation of Ag: get the purified MIL-101(Cr)-SO in 250mg embodiment 1 step (1) 3 H was added to the container, and then 20ml, 40mg / ml AgNO 3 in a solution of acetonitrile / water (volume ratio 1:1). Stir in the dark for 6 hours, centrifuge, remove the supernatant, and vacuum-dry at 120°C for 12 hours to obtain MIL-101(Cr)-SO with a loading of Ag(I) of 17.6wt%. 3 Ag.
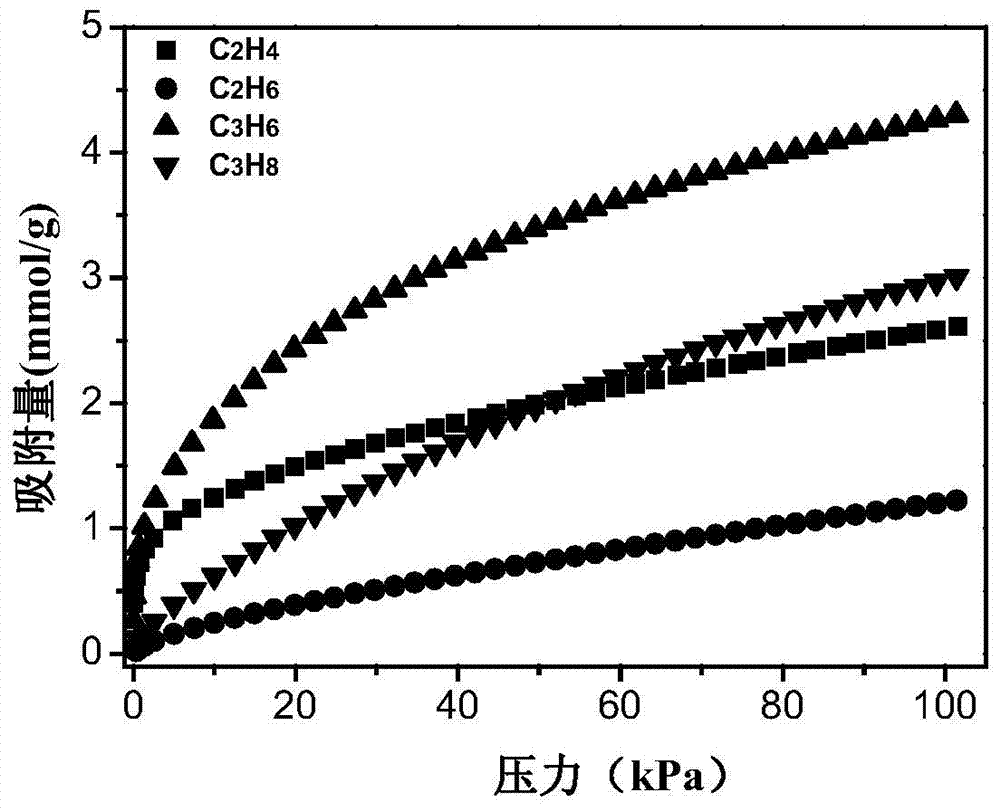
[0046] At 30°C and 100kPa, the adsorption capacity of the adsorbent for ethylene is 2.15mmol / g, and the adsorption capacity for ethane is 1.48mmol / g. AgNO can be found 3 No AgBF as active ingredient 4 The effect is good, so the next experiments all use AgBF 4 as an active ingredient.
Embodiment 3
[0048] Adsorbent MIL-101(Cr)-SO 3 The preparation of Ag: get the purified MIL-101(Cr)-SO in 250mg example 1 step (1) 3 Add H into the container, then add 20ml, 40mg / ml AgBF 4 in a solution of acetonitrile / water (volume ratio 1:1). Stir in the dark for 12 hours, centrifuge, remove the supernatant, and dry in vacuum at 120°C for 12 hours to obtain MIL-101(Cr)-SO with a loading of Ag(I) of 24.8wt%. 3 Ag.
[0049] At 30°C and 100kPa, the adsorption capacity of the adsorbent to ethylene is 2.43mmol / g, and the adsorption capacity to ethane is 1.37mmol / g, calculated by IAST C 2 h 4 / C 2 h 6 (1:1) separation selectivity is 12.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com