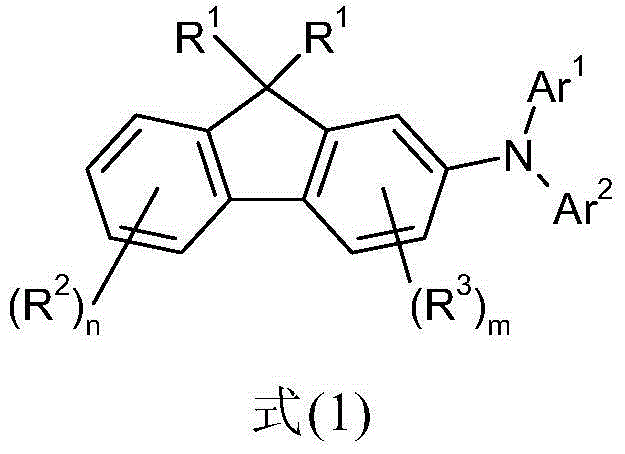
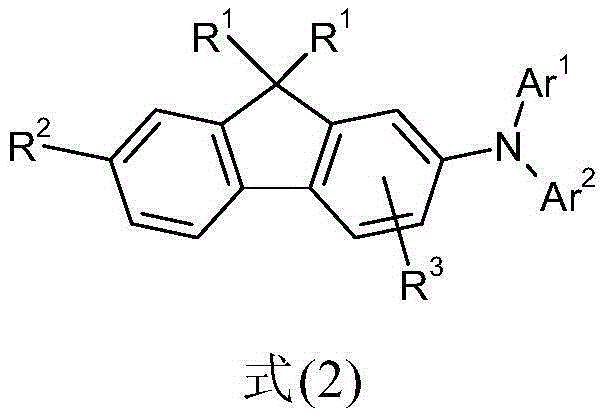
Derivatives of 2-diarylaminofluorene and organic electronic compounds containing them
An organic and radical technology, applied in the field of organic compounds, can solve problems such as poor performance data and high operating voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0241] Compound biphenyl-2-ylbiphenyl-4-yl-(9,9-dimethyl-9H-fluoren-2-yl)amine (1-1) and compounds (1-2) to (1-5) Synthesis
[0242]
[0243] 23.5 g of biphenyl-2-ylbiphenyl-4-ylamine (73 mmol) and 20.0 g of 2-bromofluorene (73 mmol) were dissolved in 500 ml of toluene: the solution was degassed and washed with N 2 saturation. Then 2.52 g (2.93 mmol) of tri-tert-butylphosphine and 0.33 g (1.46 mmol) of palladium(II) acetate were added. Subsequently 10.8 g of sodium tert-butoxide (110 mmol) were added. The reaction mixture was heated at boiling under a protective atmosphere for 6 hours. The mixture was then partitioned between toluene and water, and the organic phase was washed three times with water, washed with Na 2 SO 4 Dry and evaporate in a rotary evaporator. After filtration of the crude product with toluene through silica gel, the residue that remains is recrystallized from heptane / toluene and finally sublimed in high vacuum. The purity is 99.9%. Yield 32.0 g ...
Embodiment 2
[0248] Compound biphenyl-2-ylbiphenyl-4-yl-(9,9-diphenyl-9H-fluoren-3-yl)amine (2-1) and compounds (2-2) to (2-10) Synthesis
[0249]
[0250] 2-Bromo-9,9-diphenyl-9H-fluorene (2-1)
[0251] 30 g (103 mmol) of methyl 4'-bromobiphenyl-2-carboxylate were dissolved in 500 ml of dry THF in a flask which had been dried by heating. The clear solution was cooled to -10°C and then 102 ml (307 mmol) of freshly prepared 3 M 2-phenylmagnesium bromide solution were added. The reaction mixture was slowly warmed to room temperature and then treated with NH 4 Cl (500ml) quenched. The mixture was then partitioned between ethyl acetate and water, and the organic phase was washed three times with water, washed with Na 2 SO 4 Dry and evaporate in a rotary evaporator. 400 ml of acetic acid were carefully added to the residue. Then 80 ml of fuming hydrochloric acid were added. The batch was heated to 75°C and held at this temperature for 5 hours. During this time a white solid precipit...
Embodiment 3
[0261] Compound biphenyl-4-ylbiphenyl-2-yl-(9,9-dimethyl-7-phenyl-9H-fluoren-2-yl)amine (3-1) and compound (3-2) to Synthesis of (3-8)
[0262]
[0263] 9,9-Dimethyl-7-phenyl-9H-fluorene
[0264] Suspend 8.9 g (73 mmol) of phenylboronic acid and 20 g (73 mmol) of 2-bromo-9,9'-dimethyl-9H-fluorene in 330 ml of dimethoxyethane and 110 ml of 2 M Na 2 CO 3 in solution. To this suspension was added 2.54 g (2.0 mmol) tetrakis(triphenylphosphine)palladium. The reaction mixture was heated at reflux for 16 hours. After cooling, the reaction mixture was diluted with ethyl acetate and the organic phase was separated off, washed three times with 100 ml of water and then evaporated to dryness. The crude product was filtered through silica gel using heptane / ethyl acetate (20:1 ) to yield 18.8 g (95%) of 9,9-dimethyl-7-phenyl-9H-fluorene.
[0265] The following fluorenes were prepared analogously.
[0266]
[0267] 2-Bromo-9,9-dimethyl-7-phenyl-9H-fluorene
[0268] Dissolve 29....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com