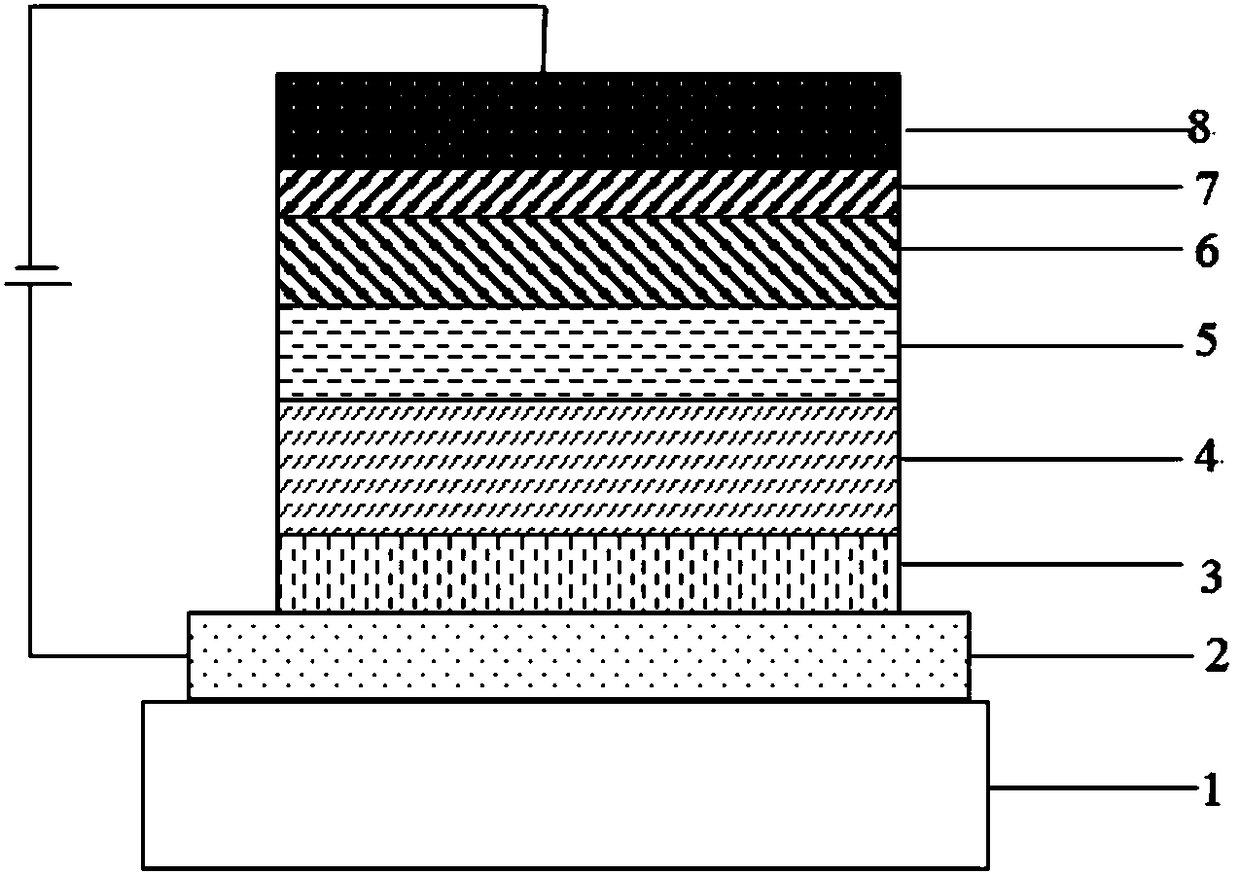
Compound taking pyridine as core and application thereof to organic electroluminescence device
A compound, azabenzene technology, applied in the direction of electrical solid-state devices, electrical components, luminescent materials, etc., can solve the problems of efficiency roll-off, low S1 state radiation transition rate, difficult exciton utilization rate and high fluorescence radiation efficiency, etc. Achieve the effects of reducing difficulty and cost, good film formation and fluorescence quantum efficiency, and avoiding aggregation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
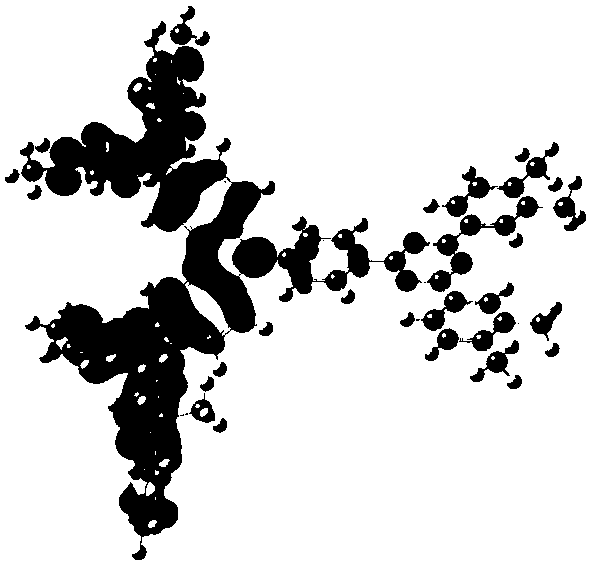
[0072] The synthesis of embodiment 1 compound 2
[0073]
[0074] The concrete synthetic route of this compound is provided now:
[0075]
[0076] In a 250ml four-neck flask, under a nitrogen atmosphere, add 0.01mol 4-(4-bromophenyl)-2,6-diphenyl-pyrimidine, 0.015mol (9,9-dimethyl-9H- Fluoren-2-yl)-(6-methyl-9H-carbazol-3-yl)-phenyl-amine, 0.03mol sodium tert-butoxide, 1×10 -4 mol Pd 2 (dba) 3 , 1×10 -4 mol of tri-tert-butylphosphine, 150ml of toluene, heated to reflux for 24 hours, sampling plate, showing no bromide remaining, the reaction was complete; naturally cooled to room temperature, filtered, and the filtrate was rotary evaporated under reduced pressure (-0.09MPa, 85°C), After passing through a neutral silica gel column, the target product was obtained with a purity of 99.20% and a yield of 59.20%.
[0077] HPLC-MS: The theoretical value is 786.37, and the measured value is 786.42.
Embodiment 2
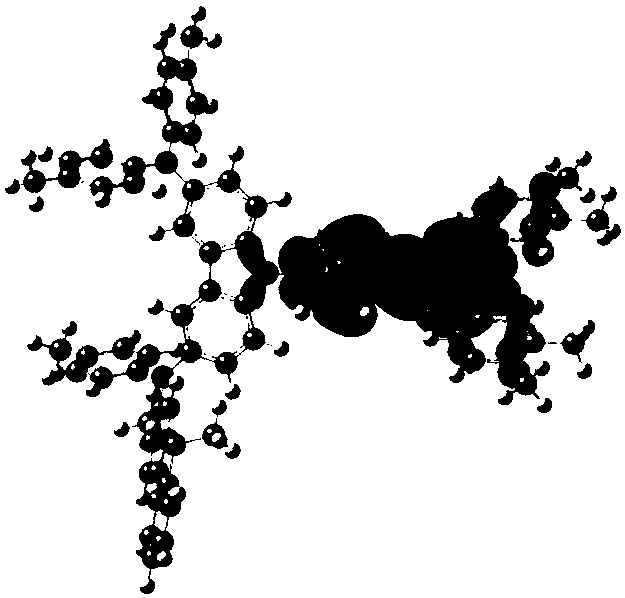
[0078] The synthesis of embodiment 2 compound 4
[0079]
[0080] The concrete synthetic route of this compound is provided now:
[0081]
[0082] In a 250ml four-neck flask, under a nitrogen atmosphere, add 0.01mol 4-(4-bromophenyl)-2,6-diphenyl-pyrimidine, 0.015mol (9,9-dimethyl-9H- Fluoren-2-yl)-(6-cyclohexyl-9H-carbazol-3-yl)-phenyl-amine, 0.03mol sodium tert-butoxide, 1×10 -4 mol Pd 2 (dba) 3 , 1×10 -4mol of tri-tert-butylphosphine, 150ml of toluene, heated to reflux for 24 hours, sampling plate, showing no bromide remaining, the reaction was complete; naturally cooled to room temperature, filtered, and the filtrate was subjected to vacuum rotary evaporation (-0.09MPa, 85°C), After passing through a neutral silica gel column, the target product was obtained with a purity of 98.89% and a yield of 48.50%.
[0083] HPLC-MS: The theoretical value is 854.43, and the measured value is 854.56.
Embodiment 3
[0084] The synthesis of embodiment 3 compound 21
[0085]
[0086] The concrete synthetic route of this compound is provided now:
[0087]
[0088] In a 250ml four-necked flask, add 0.01mol 4-(4-bromophenyl)-2,6-bis-(4-methyl-phenyl)-pyridine, 0.015molN-(9, 9-Dimethyl-9H-fluoren-2-yl)-N,N',N'-triphenyl-9H-carbazole-3,6-diamine, 0.03mol sodium tert-butoxide, 1×10 -4 mol Pd 2 (dba) 3 , 1×10 -4 mol of tri-tert-butylphosphine, 150ml of toluene, heated to reflux for 24 hours, sampling plate, showing no bromide remaining, the reaction was complete; naturally cooled to room temperature, filtered, and the filtrate was subjected to vacuum rotary evaporation (-0.09MPa, 85°C), After passing through a neutral silica gel column, the target product was obtained with a purity of 99.50% and a yield of 58.50%.
[0089] HPLC-MS: The theoretical value is 950.43, and the measured value is 950.52.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com