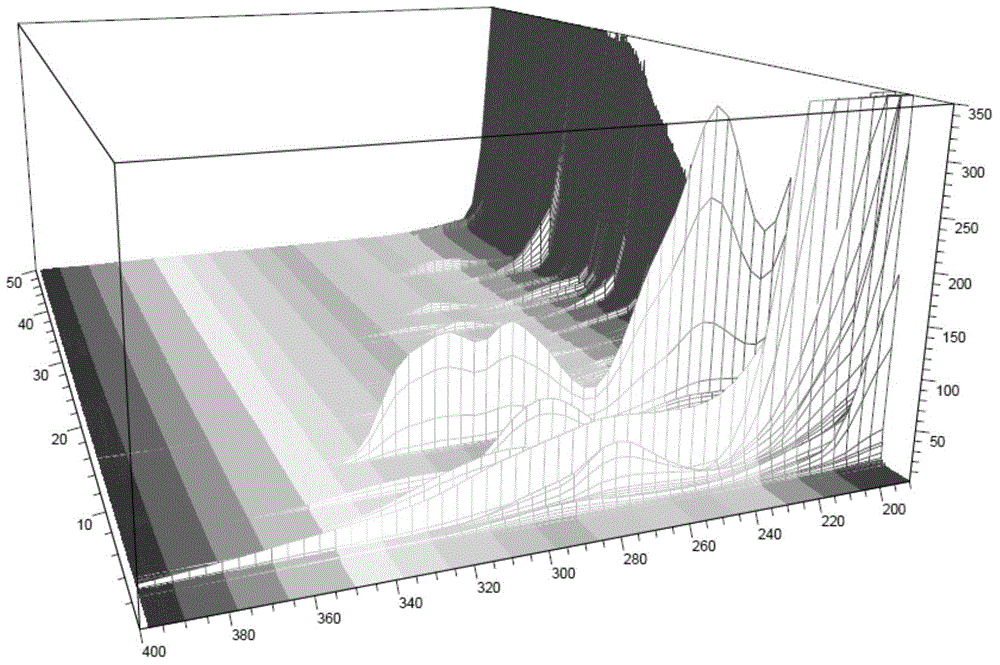
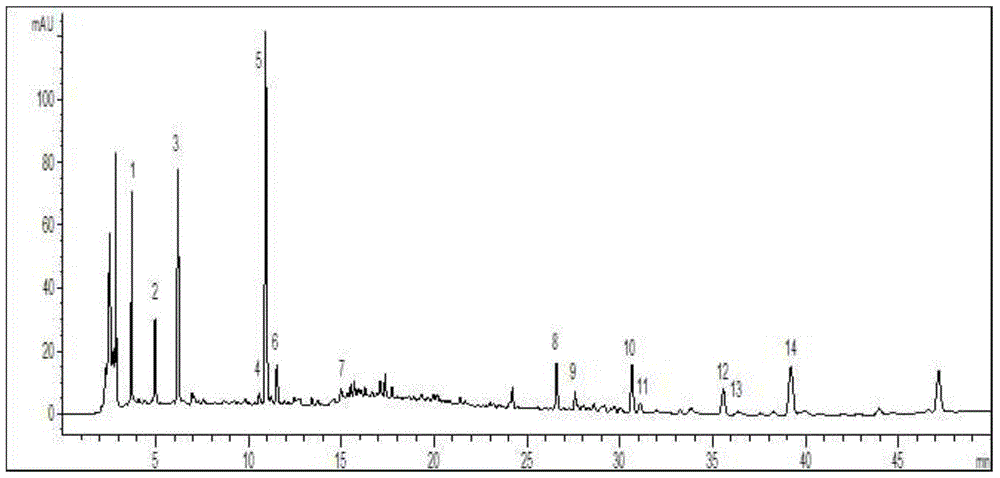
Construction method of standard finger-print and characteristic spectrum of Suoquan preparation and quality detection method
A standard fingerprint and feature map technology, which is applied in the construction method and quality inspection field of the standard fingerprint map and feature map of Shuquan preparations, can solve the problems such as the inability to effectively control the production process and product quality, and the inability to better guarantee the clinical efficacy. , to increase the quality coverage, improve detection sensitivity and detection quality, and achieve the effect of good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Example 1 Establishment of the fingerprint of Shuquan preparation. (Take Shuquan Capsules as an example)
[0064] (1) Instruments and reagents
[0065] Agilent 1260 high performance liquid chromatography, quaternary pump (G-1311C, including online vacuum degassing machine), automatic sampler (G-1329B), intelligent column oven (G-1316A), DAD detector (G -1315B), ChemStation for LC 3D systems chromatographic workstation (Agilent Technologies Co., Ltd.); SB-5200D ultrasonic cleaner (Ningbo Xinzhi Biotechnology Co., Ltd.); FAZ004B analytical balance (Shanghai Youke); BT125D electronic balance (Sai Doris Scientific Instruments (Beijing) Co., Ltd.).
[0066] (2) Selection of chromatographic conditions
[0067] ①Selection of mobile phase
[0068] Select acetonitrile-water, acetonitrile-0.1% phosphoric acid solution, acetonitrile-0.2% phosphoric acid solution, acetonitrile-0.1% formic acid solution, methanol-0.1% phosphoric acid solution, methanol-0.1% formic acid solution,...
Embodiment 2
[0115] Example 2 Establishment of the fingerprint of the intermediate of Shuquan preparation
[0116] (1) Instruments and reagents
[0117] Agilent 1260 high performance liquid chromatography, quaternary pump (G-1311C, including online vacuum degassing machine), automatic sampler (G-1329B), intelligent column oven (G-1316A), DAD detector (G -1315B), ChemStation for LC 3D systems chromatography workstation (Agilent Technologies Co., Ltd.); SB-5200D ultrasonic cleaner (Ningbo Xinzhi Biotechnology Co., Ltd.); FAZ004B analytical balance (Shanghai Youke); Sartorius Scientific Instruments (Beijing) Co., Ltd.).
[0118] (2) Chromatographic conditions
[0119] The column is Agilent ZORBAX SB-C 18 (5 μ m, 4.6 * 250mm) reversed-phase chromatographic column; Column temperature is 30 ℃; Three wavelengths of 280, 260, 230nm are switched as detection wavelength; Mobile phase A is acetonitrile, mobile phase B is 0.1% phosphoric acid solution, and the total flow rate is 1.0ml / min; Analysi...
Embodiment 3
[0154] Example 3 Establishment of fingerprints of Shuquan preparations (taking Shuquan granules as an example).
[0155] (1) Instruments and reagents
[0156] Agilent 1260 high performance liquid chromatography, quaternary pump (G-1311C, including online vacuum degassing machine), automatic sampler (G-1329B), intelligent column oven (G-1316A), DAD detector (G -1315B), ChemStation for LC 3D systems chromatographic workstation (Agilent Technologies Co., Ltd.); SB-5200D ultrasonic cleaner (Ningbo Xinzhi Biotechnology Co., Ltd.); FAZ004B analytical balance (Shanghai Youke); BT125D electronic balance (Sai Doris Scientific Instruments (Beijing) Co., Ltd.).
[0157] (2) Chromatographic conditions
[0158] The column is Thermo Syncronis-C 18 (5 μ m, 4.6 * 250mm) reversed-phase chromatographic column; Column temperature is 35 ℃; With 280, 260, 230nm three wavelengths are switched as detection wavelength; Mobile phase A is acetonitrile, and mobile phase B is 0.05% phosphoric acid sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com