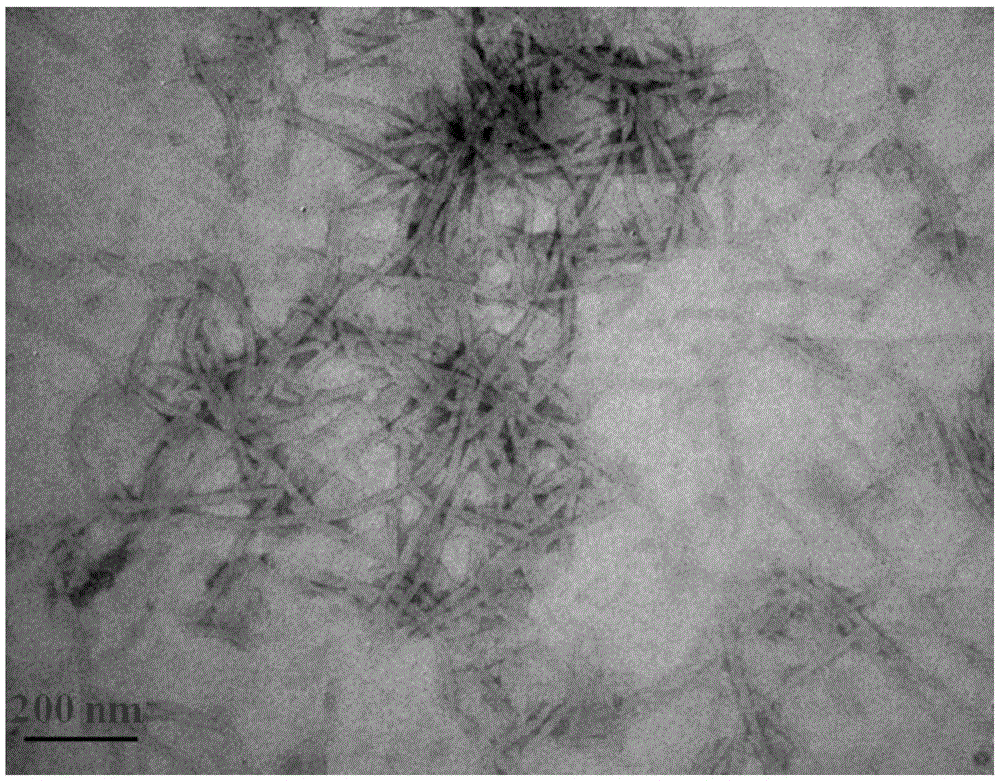
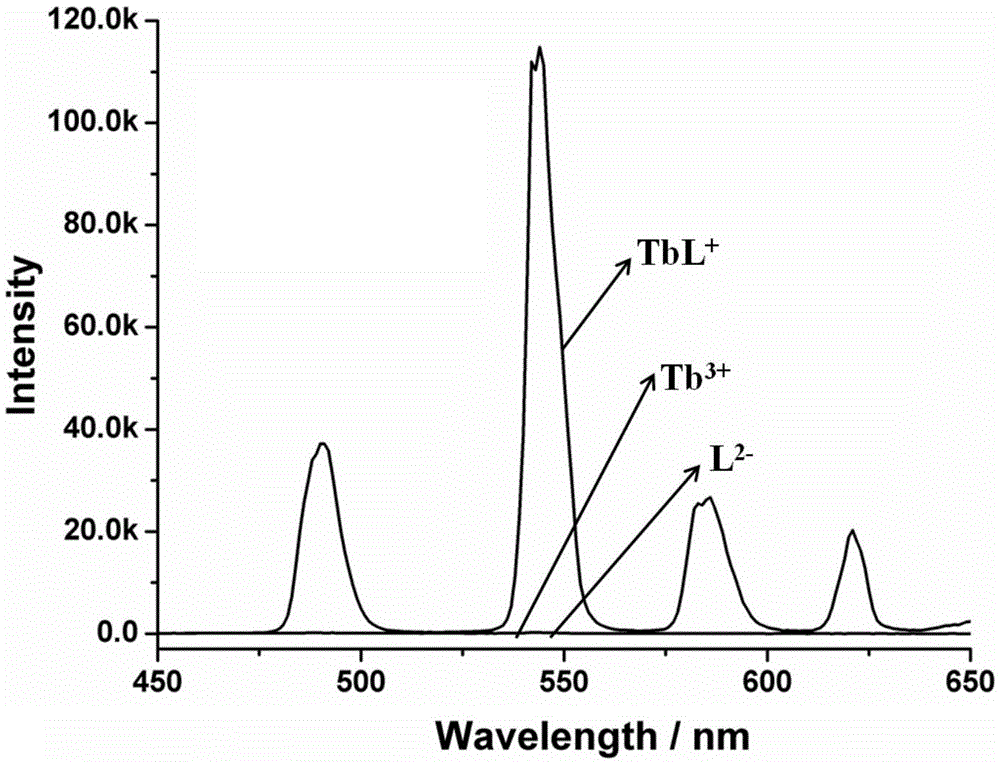
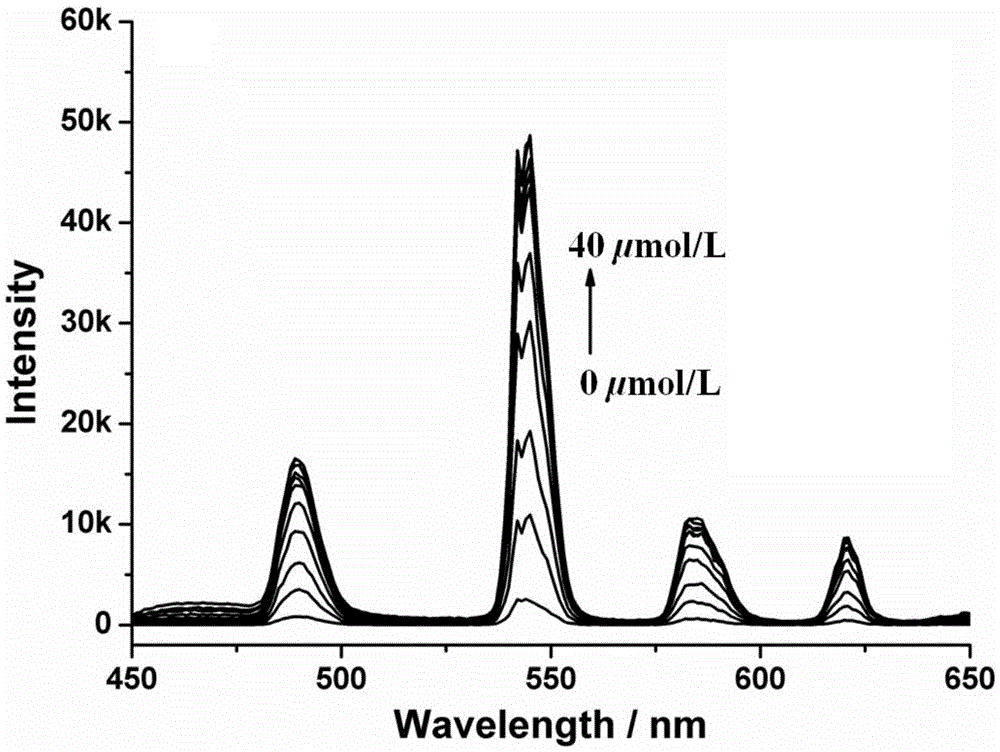
Amphiphilic tb(Ⅲ) complexes, preparation methods thereof, and preparation methods and applications of fluorescent nanofibers
A technology of fluorescent nanofibers and complexes, applied in the direction of steroids, fluorescence/phosphorescence, chemical instruments and methods, etc., to achieve the effects of mild reaction conditions, simple preparation methods, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] (1) Synthesis of 6-hydroxymethyl-2-formic acid ethyl pyridine
[0051] Under a nitrogen gas flow rate of 1 mL / s, 5 g of ethyl 2,6-dicarboxylate pyridine and 0.424 g of sodium borohydride were added to a round-bottomed flask filled with 95 mL of absolute ethanol, and ethyl 2,6-dicarboxylate The molar ratio of pyridine and sodium borohydride is 1:0.5. Reflux at 90°C for 5 hours, then adjust the pH value to 5, extract with chloroform, dry over anhydrous sodium sulfate, remove chloroform by rotary evaporation, and use a mixture of methanol and chloroform at a volume ratio of 1:15 Use the mobile phase and silica gel as the stationary phase for column chromatography to obtain 6-hydroxymethyl-2-formic acid ethyl pyridine.
[0052] 1 H-NMR, δ H (400MHz, CDCl 3 , Me 4 Si): 1.43 (3H, O-CH 2 - CH 3 ), 4.47 (2H, O- CH 2 -CH 3 ), 4.87(2H, CH 2 -OH), 7.52 (1H, pyridineprotons), 7.85 (1H, pyridineprotons), 8.03 (1H, pyridineprotons). Its reaction equation is as follow...
Embodiment 2
[0078] (1) Synthesis of 6-hydroxymethyl-2-formic acid ethyl pyridine
[0079] Under a nitrogen gas flow rate of 1 mL / s, 5 g of ethyl 2,6-dicarboxylate pyridine and 0.505 g of sodium borohydride were added to a round-bottomed flask filled with 125 mL of absolute ethanol, and ethyl 2,6-dicarboxylate The molar ratio of pyridine and sodium borohydride is 1:0.6, reflux reaction at 90°C for 5 hours, then adjust the pH to about 5, extract with chloroform, dry over anhydrous sodium sulfate, remove chloroform by rotary evaporation, and use methanol and tris The mixed liquid with a volume ratio of 1:15 of methyl chloride was used as the mobile phase, and silica gel was used as the stationary phase for column chromatography to obtain 6-hydroxymethyl-2-formic acid ethyl pyridine. 1 H-NMR, δ H (400MHz, CDCl 3 , Me 4 Si): 1.43 (3H, O-CH 2 - CH 3 ), 4.47 (2H, O- CH 2 -CH 3 ), 4.87(2H, CH 2 -OH), 7.52 (1H, pyridineprotons), 7.85 (1H, pyridineprotons), 8.03 (1H, pyridineprotons)....
Embodiment 3
[0096] (1) Synthesis of 6-hydroxymethyl-2-formic acid ethyl pyridine
[0097] Under the condition of nitrogen gas with a flow rate of 1.2mL / s, 5g of ethylpyridine 2,6-dicarboxylate and 0.509g of sodium borohydride were added to a round-bottomed flask filled with 130mL of absolute ethanol, and ethyl 2,6-dicarboxylate The molar ratio of ester pyridine to sodium borohydride is 1:0.7. Reflux reaction at 90°C for 5h, then adjust the pH to 5, extract with chloroform, dry over anhydrous sodium sulfate, remove chloroform by rotary evaporation, and use methanol and tris The mixed liquid with a volume ratio of 1:15 of methyl chloride was used as the mobile phase, and silica gel was used as the stationary phase for column chromatography to obtain 6-hydroxymethyl-2-formic acid ethyl pyridine. 1 H-NMR, δ H (400MHz, CDCl 3 , Me 4 Si): 1.43 (3H, O-CH 2 - CH 3 ), 4.47 (2H, O- CH 2 -CH 3 ), 4.87(2H, CH 2 -OH), 7.52 (1H, pyridineprotons), 7.85 (1H, pyridineprotons), 8.03 (1H, pyri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com