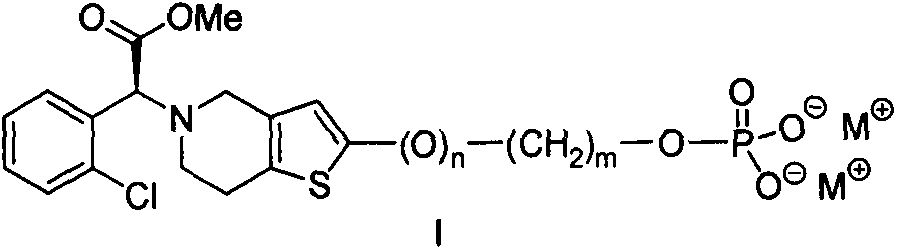
Preparation method and medical application of water-soluble 2-hydroxyl tetrahydrothienopyridine derivatives
A solvate and drug technology, which is applied to the preparation of water-soluble 2-hydroxytetrahydrothienopyridine derivatives and the field of medical application thereof, and can solve the problems of slow onset, poor water solubility and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
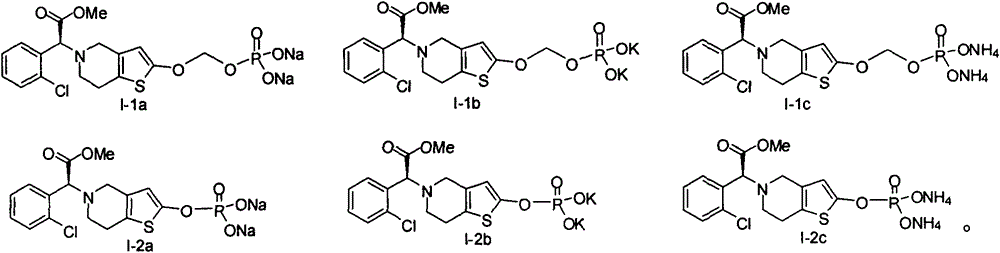
[0079] (S)-(5-(1-(2-chlorophenyl)-2-methoxy-2-carbonylethyl)-4,5,6,7-tetrahydrothieno[3,2-c] Preparation of Sodium Pyridin-2-yloxy)methylphosphate (Formula I-1a Compound)
[0080]
[0081] Step 1: Di-tert-butyl (5-trityl-4,5,6,7-tetrahydrothieno[3,2-c]pyridin-2-yloxy)methylphosphate (formula XII-1 compound) preparation
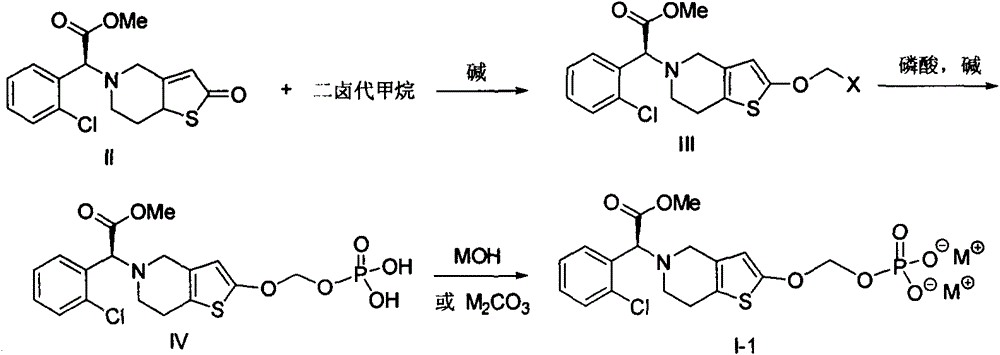
[0082] Under nitrogen protection, 5-trityl-5,6,7,7a-tetrahydrothieno[3,2-c]pyridin-2(4H)-one (compound of formula X, 3.98g, 10mmol) and sodium iodide (100mg) were added into anhydrous tetrahydrofuran (50mL), and a solution of sodium hexamethyldisilazide in tetrahydrofuran (1M, 11.0mL, 11.0mmol) was added dropwise at 0°C. Stir at room temperature for 1 hour, then add di-tert-butyl chloromethyl phosphate (compound of formula XI-1, 3.10 g, 12 mmol), continue to stir at room temperature for 30 minutes, then heat to 80 ° C, keep warm until the raw material disappears (TLC detection ). The solvent was evaporated under reduced pressure, water (50 mL) was added...
Embodiment 2
[0089] (S)-(5-(1-(2-chlorophenyl)-2-methoxy-2-carbonylethyl)-4,5,6,7-tetrahydrothieno[3,2-c] Preparation of Sodium Pyridin-2-yloxy)methylphosphate (Formula I-1a Compound)
[0090]
[0091] Step 1: (S)-2-(2-(Chloromethoxy)-6,7-dihydrothieno[3,2-c]pyridin-5(4H)-yl)-2-(2-chloro The preparation of phenyl) methyl acetate (formula III-1 compound)
[0092] Under nitrogen protection, (2S)-2-(2-oxo-7,7a-dihydrothieno[3,2-c]pyridin-5(2H,4H,6H)-yl)-2-(2 -Chlorophenyl)-methyl acetate (compound of formula II, 3.38g, 10mmol) (prepared with reference to the method described in Chinese patent application 201010624329.7), chlorobromomethane (10g, 78mmol), potassium iodide (50mg) and potassium tert-butoxide (1.34 g, 12 mmol) was added into tetrahydrofuran (50 mL), stirred at room temperature for 1 hour, then heated to 40° C., and kept warm until the raw material disappeared (TLC detection). The solvent was evaporated under reduced pressure, water (50 mL) was added to the residue, extracte...
Embodiment 3
[0096] (S)-5-(1-(2-chlorophenyl)-2-methoxy-2-carbonylethyl)-4,5,6,7-tetrahydrothieno[3,2-c]pyridine Preparation of -2-base sodium phosphate (compound of formula I-2a)
[0097]
[0098] Step 1: Preparation of 5-trityl-4,5,6,7-tetrahydrothieno[3,2-c]pyridin-2-yl di-tert-butyl phosphate (compound of formula XVI-1)
[0099] 5-trityl-5,6,7,7a-tetrahydrothieno[3,2-c]pyridin-2(4H)-one (compound of formula X, 3.98g, 10mmol), DBU (3.3mL , 22mmol) and DMAP (0.12g, 1mmol) were dissolved in anhydrous tetrahydrofuran (30mL), and the phosphorylation reagent formula XV-1 compound (5.65g, 20.75mmol) (prepared according to the method described in WO2005063777) was added dropwise at 0°C Tetrahydrofuran (5 mL) solution was added after dropping, slowly warmed to room temperature and stirred overnight. Add saturated sodium bicarbonate solution (5mL) to quench the reaction, evaporate the organic solvent under reduced pressure, add water (20mL) to the residue, extract with ethyl acetate (50mL x...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com