A kind of preparation method of myoglobin detection reagent
A technology for detecting reagents and myoglobin, which is applied in the field of in vitro diagnostic reagents for medical devices, can solve problems such as elevated levels and poor specificity, and achieve high analytical sensitivity and high specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
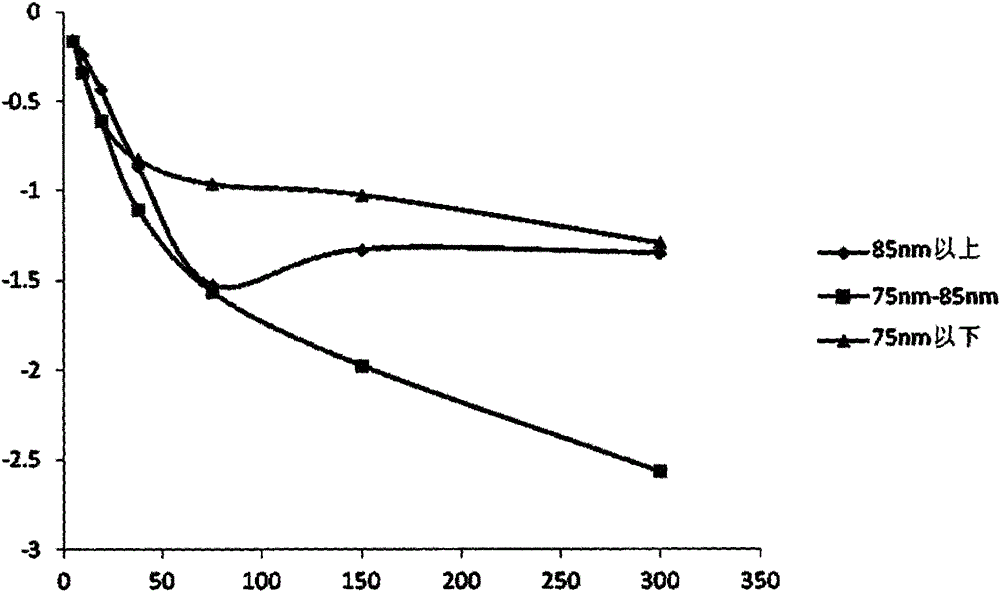
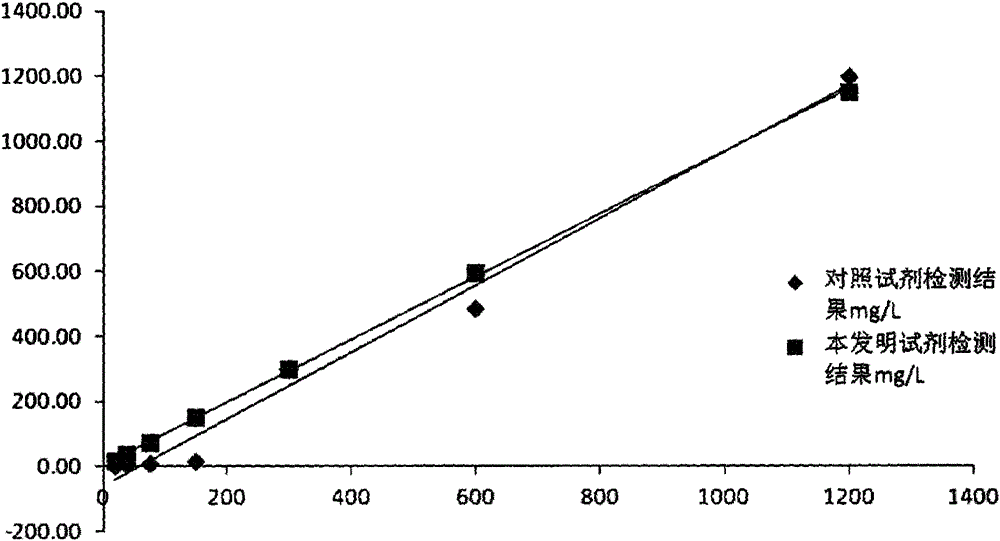
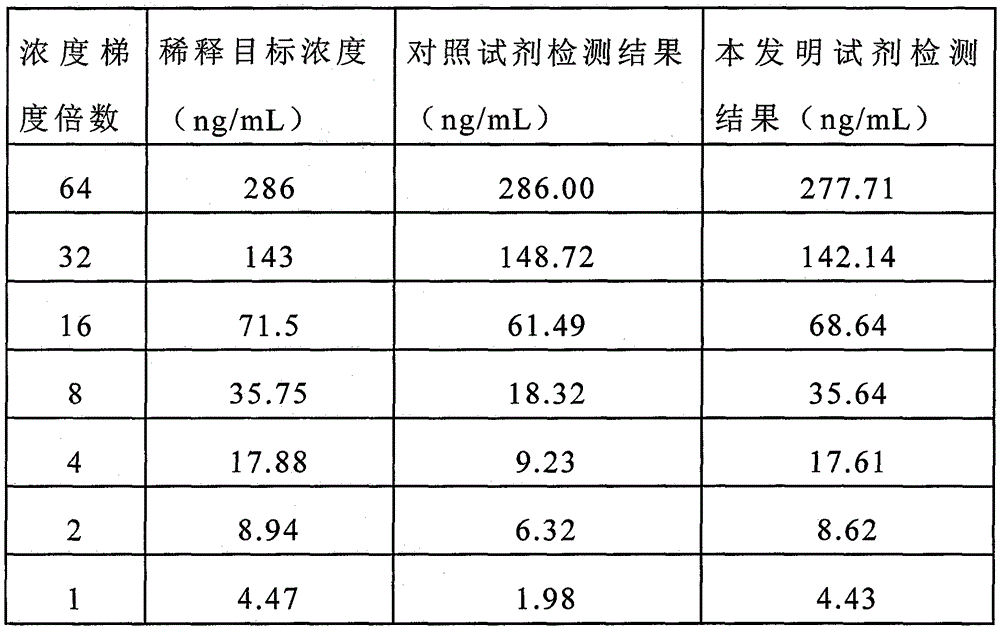
Image
Examples
preparation example Construction
[0047] The preparation method of the myoglobin detection reagent is as follows:
[0048] 1), adding the coagulant, BSA and preservative to the buffer solution to prepare reagent I;
[0049] 2), heating and boiling chloroauric acid and trisodium citrate according to the mass ratio of 1:1 to obtain a colloidal gold solution; adding the MYO antibody to the colloidal gold solution, and adding stabilizers, buffers and preservatives to obtain a reagent II; Wherein, when configuring chloroauric acid and trisodium citrate solution, use 0.2 μm filter membrane to filter;
[0050] 3) Reagent I and Reagent II are used together at a volume ratio of 5:1 to obtain a myoglobin detection reagent.
Embodiment 1
[0053] Preparation of colloidal gold particles (all the following operations need to be completed in a high cleanliness and dust-free space)
[0054] 1) Preparatory work: all the glass containers used are first washed with detergent and rinsed with running water, then soaked in siliconized reagent overnight, siliconized the inner surface of the goldware used, rinsed with distilled water, and set aside;
[0055] 2) Prepare a solution of 1% (w / v) chloroauric acid and 1% (w / v) sodium citrate, and filter it with a 0.2 μm filter membrane;
[0056] 3) Put a magnetic stirrer in a cleaned 1000ml round bottom flask, and add 1000ml ultrapure water;
[0057] 4) Add a certain volume concentration of 1% (w / v) chloroauric acid, stir at a high speed around 600rpm, and heat until the solution boils, then quickly add a certain volume concentration of 1% sodium citrate solution into the flask and keep heating and vigorously stirred for 10 min, the solution color first turned black, and then gr...
Embodiment 2
[0061] Preparation of reagent one
[0062] The formula of reagent one is as follows: Tris-HCl buffer solution, 0.9% NaCl (w / v), 2% BSA (w / v), 0.1% NaN3 (w / v), 0.05% Tween20 (w / v), according to The concentration of the Tris quality control buffer was adjusted according to the amount of HCl used to adjust the pH of the buffer.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com