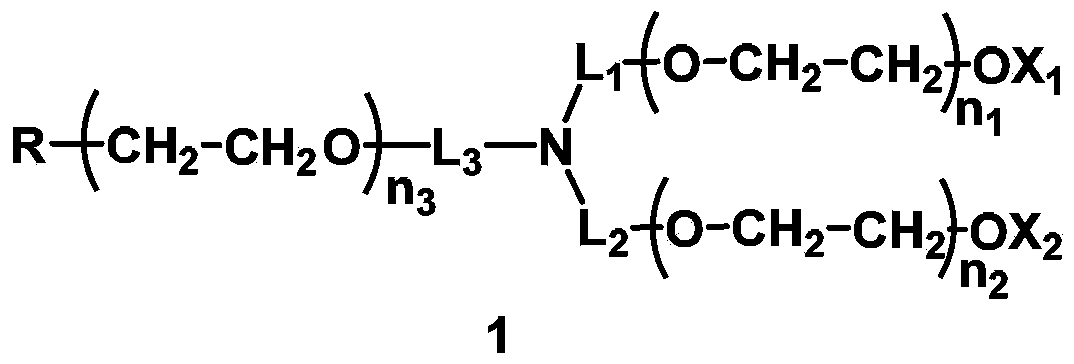
Single functionalized polyethylene glycol with nitrogen-atom branching center, preparation method and biologically-relevant substance thereof
A technology of polyethylene glycol and functionalization, which is applied in the field of preparation methods and biologically related substances, monofunctionalized polyethylene glycol, and can solve the problem of limiting the type and use of branched polyethylene glycol, and the low reactivity of carbon atoms , limit the preparation of small molecule initiators, etc., to achieve the effect of enriching types, strengthening protection, and improving the state
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0069] 1.1 Preparation of intermediate compound (3)
[0070] The intermediate compound (3) of the present invention can be produced as follows. 2 to 2000 times the molar amount of ethylene oxide is polymerized with the terminal hydroxyl-protected compound (4) containing two exposed hydroxyl groups, and an excess of deprotonating reagent is added to generate polyethylene glycol with two branched chains Anion intermediate (5); Hydrocarbyl X for terminal oxyanions 1 、X 2 The intermediate compound (6) is obtained by etherification and capping; the hydroxyl group at the end of the main chain is deprotected; after the newly formed hydroxyl group at the end of the main chain initiates the polymerization of ethylene oxide, the intermediate compound (3) can be obtained by adding a proton source. (i.e. steps a~d above).
[0071] 1.1.1 Preparation of polyethylene glycol anion intermediate (5) (step a)
[0072] The preparation of intermediate (5) includes two steps: the polymerization...
Embodiment 1
[0332] Embodiment 1: R is the preparation of monofunctional branched polyethylene glycol when class H
[0333] Preparation of Compound H1-1
[0334] In this example, the H-like compound selected L 1 =CH 2 CH 2 , L 2 =CH 2 CH 2 , L 3 =CH 2 CH 2 ,X 1 =X 2 =CH 3 , q=0, the protecting group PG=TBS of the terminal hydroxyl group of the main chain of the small molecule initiator. The total molecular weight is designed to be about 20000, and the molecular weight of the two branched chains is about 2*8500=17000, namely n 1 ≈n 2 ≈193; the molecular weight of the main chain containing active functional groups is about 3000, that is, n 3 ≈68.
[0335]
[0336] a. Add tetrahydrofuran (250mL), small molecule initiator (2.532mmol) and diphenylmethyl potassium (4.0mmol) sequentially into an anhydrous and oxygen-free airtight reaction kettle;
[0337] b. Add the calculated amount of ethylene oxide (50mL), gradually raise the temperature to 60°C, and react for 48 hours;
[...
Embodiment 2
[0435] The preparation of embodiment 2 active ester derivatives
[0436] Synthesis of Active Ester A1-1
[0437] Synthesis of active ester (A1-1), where L 1 =CH 2 CH 2 , L 2 =CH 2 CH 2 , L 3 =CH 2 CH 2 ,X 1 =X 2 =CH 3 , q=0, the molecular weight is about 20000, where n 1 , n 2 , n 3 The value of is the same as that of compound H1-1. In this example, the corresponding active ester was directly prepared by reacting the hydroxyl group at the end of the main chain containing the active functional group of compound H1-1 with carbonate.
[0438]
[0439] In a dry and clean 1L round bottom flask, add 40g of branched polyethylene glycol (H1-1, dewatered by azeotropic toluene) prepared in Example 1, 500mL of acetonitrile, 40mL of triethylamine and 10g of N,N' - Disuccinimidyl carbonate, reacted at room temperature for 24 hours, concentrated, and recrystallized from isopropanol to obtain the active ester (A1-1) as a white solid.
[0440] The hydrogen spectrum data of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com