Biological film inhibiting peptide and application thereof
A biofilm-inhibiting peptide and biofilm technology, applied in the field of biomedicine, can solve problems such as clinical hazards, lack of prevention and treatment methods for Staphylococcus epidermidis infection, and prolonged healing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1, synthetic biomembrane inhibitory peptide
[0020] The biomembrane inhibitory peptide (hereinafter referred to as PT-8 polypeptide) contains 8 amino acid residues, and its sequence is: Pro Tyr Gly Gly Phe Ser Phe Thr (PYGGFSFT) (SEQ ID NO.1), using artificial standard solid-phase synthesis scheme, It was carried out on a solid-phase peptide synthesizer, the solid-phase support agent was resin, and the mobile phase was dimethylformamide / dichloromethane (1:1). The polypeptide shown in SEQ ID NO.1 is synthesized through multi-step reactions. Synthesized peptides using LC6000 high performance liquid chromatography, C 18 Column (Kromasil 20×250mm C 18 120A) for purification, the product peaks were collected to obtain a PT-8 polypeptide with a purity of 96.8%, vacuum-dried, weighed, divided into 20 mg / bottles, and stored at -20°C for future use. Then mass spectrometry was carried out, and the molecular weight was 874.4Da. The results showed that ESI-MS m / z: 87...
Embodiment 2
[0021] Embodiment 2, PT-8 polypeptide antibacterial test
[0022] (1) Determination of minimum inhibitory concentration (MIC) of PT-8 polypeptide against Staphylococcus epidermidis ATCC35984 by microbroth dilution method
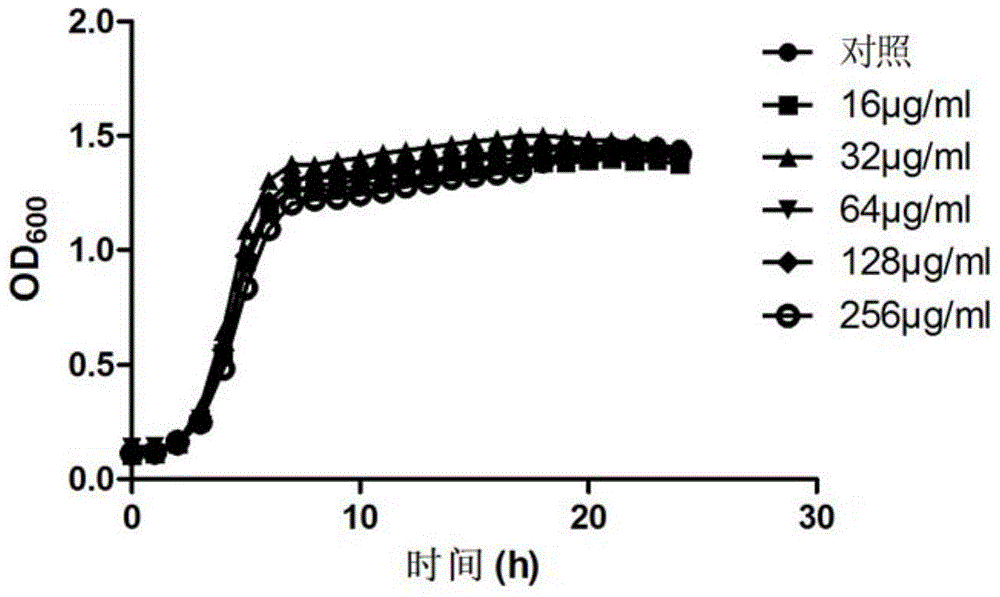
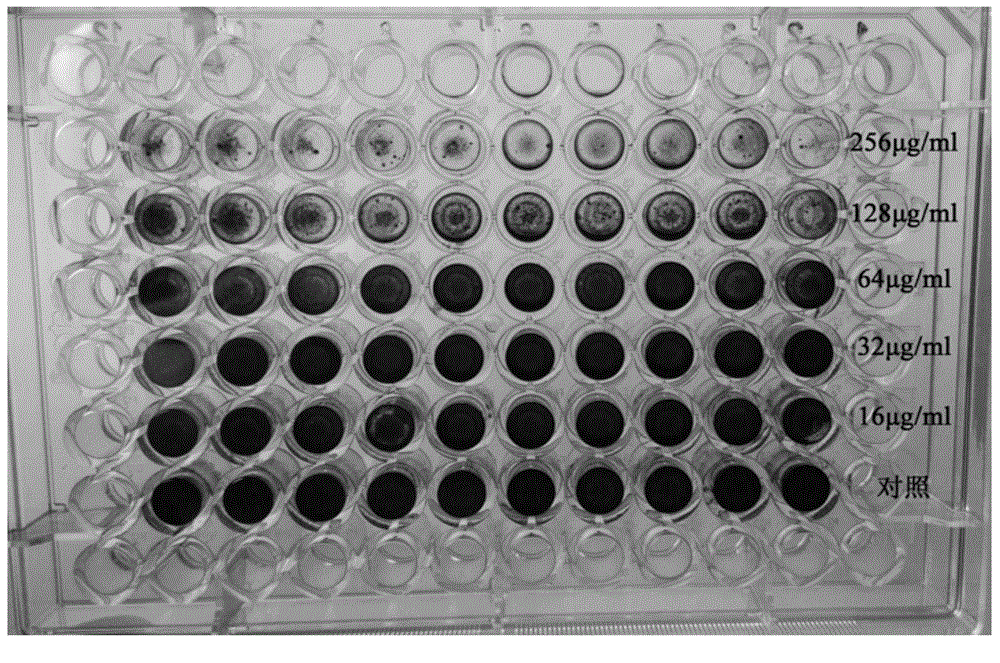
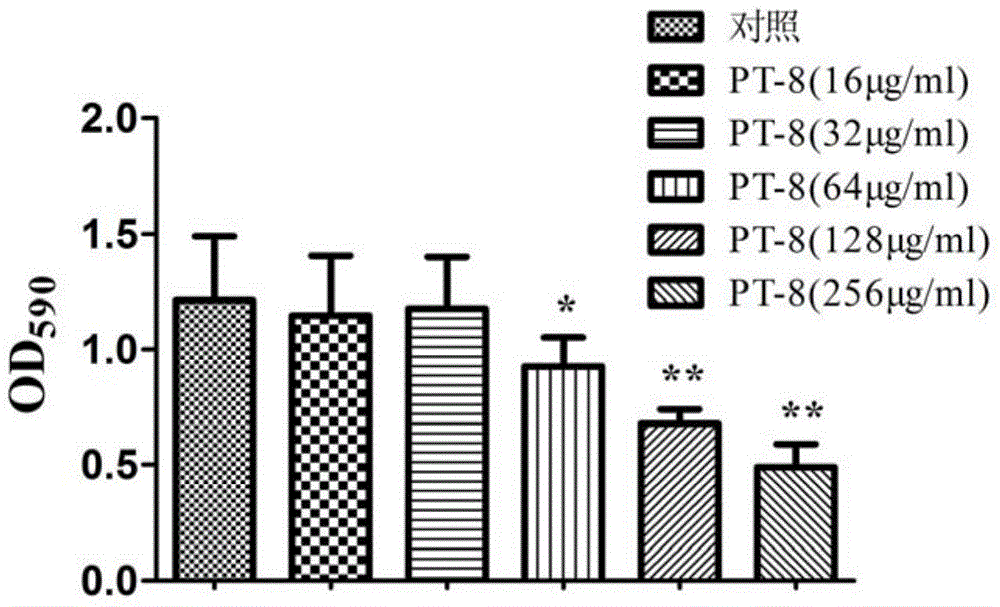
[0023] Inoculate Staphylococcus epidermidis ATCC35984 on a Columbia blood plate, culture at 37°C for 24 hours, pick a single colony in a conical flask filled with 10ml of MH broth, shake at 37°C for 24 hours, then collect the bacterial liquid and dilute it to 10 6 CFU / ml, inoculate the diluted bacterial solution in a 96-well plate, 100 μL per well, and then add the peptide synthesized in Example 1 to a final concentration of 256 μg / ml, 128 μg / ml, 64 μg / ml, and 32 μg / ml , 16 μg / ml, 8 μg / ml, 4 μg / ml, 2 μg / ml and 1 μg / ml, and control the final volume to 200 μL, MH broth was used as blank control, and then placed at 37 ° C, 5% CO 2 Cultivate under conditions for 24 hours, observe with the naked eye, take the lowest drug concentration that inhibits bacterial gr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com