Platinum (II) complex by taking chiral compound as ligand as well as synthetic method and application of platinum (II) complex
A synthesis method and technology of complexes, which are applied in the field of medicine to achieve the effects of good medicinal value and significant in vitro anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Accurately weigh 0.5mmol of R-(+)-L and 0.5mmol of dichlorobis(dimethylsulfoxide) platinum(II), dissolve R-(+)-L in 40mL of 100v In / v% methanol, dichlorobis(dimethylsulfoxide) platinum (II) was dissolved in 10mL of water, the two solutions were mixed, reacted at 80°C for 28 hours, concentrated and evaporated to remove most of the solvent (solvent 85% of the added amount), cooled to room temperature and stood still, and a dark green solid product was precipitated (yield 95%).
[0048] The obtained dark green solid product is identified:
[0049] (1), infrared spectrum, its spectrogram is as follows figure 1 shown.
[0050] IR(KBr):3406,3066,3000,2912,2346,1599,1564,1509,1457,1407,1317,1286,1202,1118,1026,944,764,691,606,521cm -1 .
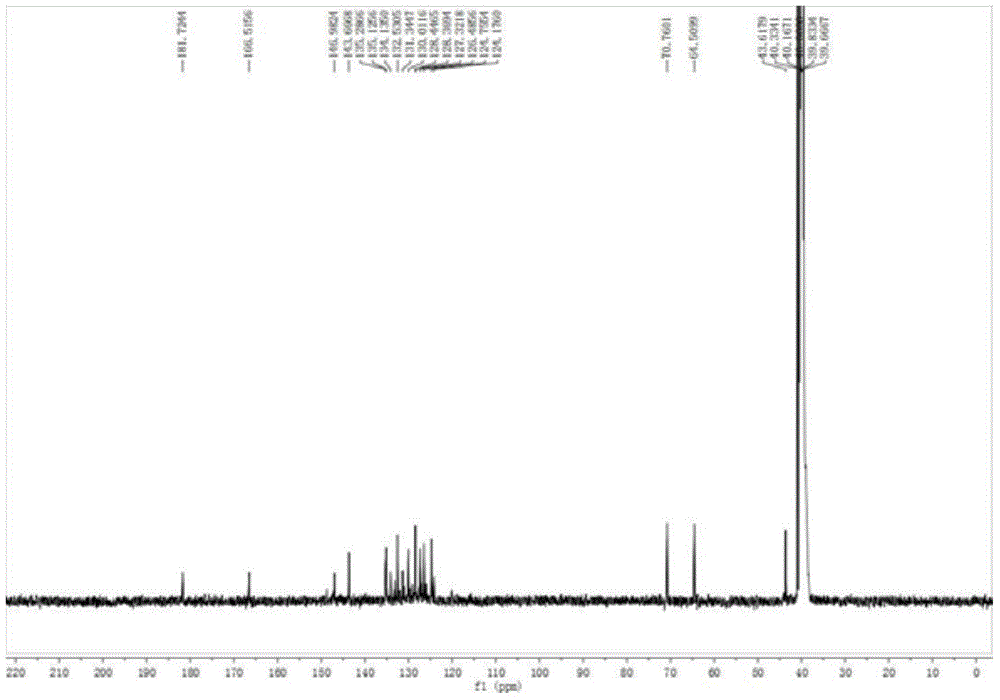
[0051] (2), proton nuclear magnetic resonance spectrogram, its spectrogram is as figure 2 shown.
[0052] 1 H NMR (500MHz, DMSO-d 6 )δ9.06(s,1H),8.93–8.91(m,1H),8.81(d,J=7.4Hz,1H),8.61(d,J=8.1Hz,1H),8.38(d,J=8.5 Hz,1H),8.33(dd,J=7....
Embodiment 2
[0064] Accurately weigh 0.5mmol of S-(–)-L and 0.5mmol of dichlorobis(dimethylsulfoxide) platinum(II), dissolve S-(–)-L in 30mL of 80v Dissolve dichlorobis(dimethylsulfoxide) platinum (II) in 25mL of 90v / v% ethanol in / v% methanol, mix the two solutions, react at 75°C for 24 hours, concentrate and evaporate to remove After most of the solvent (80% of the added amount of solvent), it was cooled to room temperature and stood still, and a dark green solid product was precipitated (yield 90%).
[0065] The obtained dark green solid product is identified:
[0066] (1), infrared spectrum, its spectrogram is as follows Figure 5 shown.
[0067] IR(KBr):3481,3736,3066,2863,2363,1997,1638,1602,1542,1473,1457,1410,1388,1353,1317,1281,1262,1218,1199,1163,1106,1095,1040 ,966,938,916,875,831,798,765,694,604,573,494cm -1 .
[0068] (2), proton nuclear magnetic resonance spectrogram, its spectrogram is as Figure 6 shown.
[0069] 1 H NMR (500MHz, DMSO-d 6 )δ9.06(s,1H),8.92–8.90(m,1...
Embodiment 3
[0082] Accurately weigh 0.5mmol of R-(+)-L and 1.5mmol of dichlorobis(dimethylsulfoxide) platinum(II), dissolve R-(+)-L in 60mL of In a mixed solution of 100v / v% methanol and 100v / v% ethanol (the volume ratio of 100v / v% methanol and 100v / v% ethanol is 6:7), dichlorobis(dimethylsulfoxide) Platinum (II) was dissolved in 30mL of a mixed solution consisting of water and 100v / v% methanol (the volume ratio of water and 100v / v% methanol was 1:99), and the two solutions were mixed and reacted at 90°C for 36 hours , concentrated and evaporated to remove most of the solvent (88% of the added amount of solvent), cooled to room temperature and left to stand, and a dark green solid product (yield 85%) was precipitated.
[0083] The obtained dark green solid product was detected by infrared spectroscopy, NMR, and electrospray mass spectrometry, and it could be determined that the obtained product was R-(+)-Pt.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com