Manganese and zinc metal complexes taking alocasia amazonica alkali as ligand as well as synthesis method and application of manganese and zinc metal complexes
A kind of technology of Guanyinlianamine base and synthesis method, which can be applied in the field of medicine and can solve the problems such as the blank of chemical synthesis and biological activity research.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
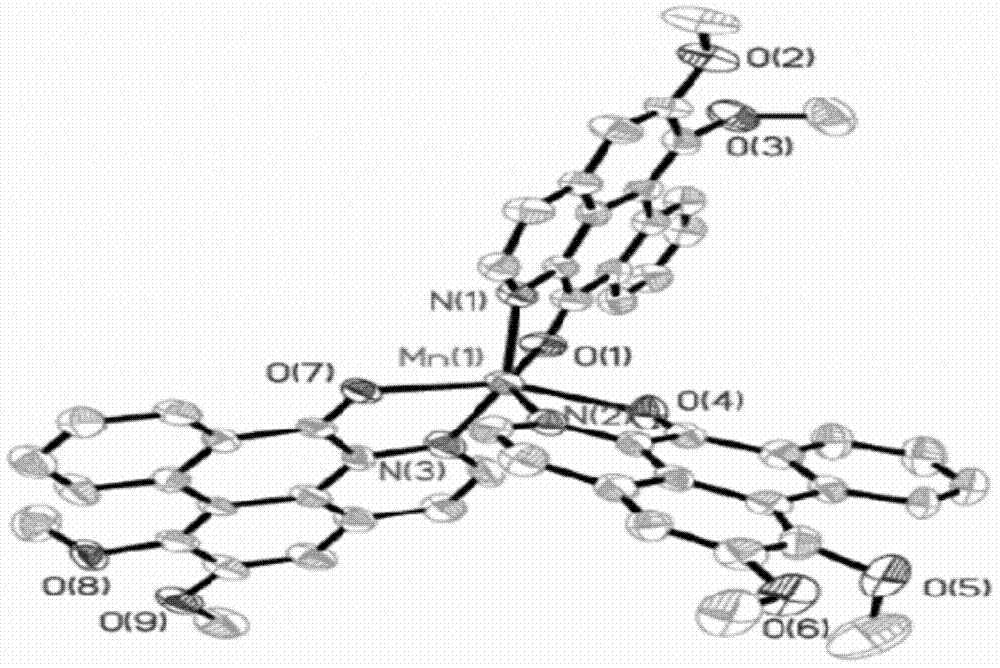
[0029] Embodiment 1: Synthesize Mn with solution method Ⅱ -LY metal complexes
[0030] 0.15mmol Mn(ClO 4 ) 2 ·6H 2 O was dissolved in 5mL dimethyl sulfoxide, 0.1mmol LY was dissolved in 10mL methanol, after the two solutions were mixed, stirred and refluxed at 65°C for 24 hours, after the reaction was completed, lowered to 25°C to obtain a red solution; Concentrate to remove most of the solvent, and a large amount of precipitates precipitate out. After standing still, the precipitates are separated out, filtered after cooling, and the obtained precipitates are washed and dried to obtain a red crystal product (yield 83%).
[0031] Infrared spectroscopy, elemental analysis, electrospray mass spectrometry, and single crystal diffraction analysis were carried out on the obtained red crystals. The specific spectral characteristics are as follows:
[0032] (1) Infrared Spectrum
[0033] IR(KBr):(Ar-H)2942(m),(C=O)1609(m),(C=C)158814801466(s),(C-O)14121269(vs),(C=N)1096( s) cm ...
Embodiment 2
[0039] Embodiment 2: Synthesize Zn with solution method Ⅱ -LY complex
[0040] 0.15mmol Zn(ClO 4 ) 2 ·6H 2 O was dissolved in 5 mL of acetone, and 0.1 mmol LY was dissolved in 10 mL of acetone. After the two solutions were mixed, they were stirred and refluxed at 80 ° C for 18 hours. After the reaction was completed, the temperature was lowered to 25 ° C to obtain a red solution; Concentrate under reduced pressure to remove most of the solvent, stand still, precipitate, and separate red crystals, wash and dry to obtain a red crystal product (yield 90%).
[0041] Infrared spectroscopy, elemental analysis, electrospray mass spectrometry, and single crystal diffraction analysis were carried out on the obtained red crystals. The specific spectral characteristics are as follows:
[0042] (1) Infrared Spectrum
[0043] IR(KBr): (Ar-H)2944(m), (C=O)1608(m), (C=C)157814821467(s), (C-O)14121275(vs), (C=N)1045( s) cm -1
[0044] (2) elemental analysis, Anal.Calc.(for C 36 h 26...
Embodiment 3
[0048] Embodiment 3: solvothermal synthesis of Mn Ⅱ -LY metal complexes
[0049] In a thick-walled glass tube open at one end, add 0.1 mmol Mn(ClO 4 ) 2 ·6H 2 O and 0.1mmolLY, then add 2mL chloroform, after freezing with liquid nitrogen, seal the open end under vacuum conditions, mix well and react at 80°C for 72 hours, remove the solvent after the reaction, take the resulting red solid and wash it with ethanol , to remove unreacted substances and impurities, and vacuum-dried at 40°C for 8 hours to obtain a red solid, which was identified as the complex [C 56 h 41 Cl 8 MnN 3 o 17 ].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com