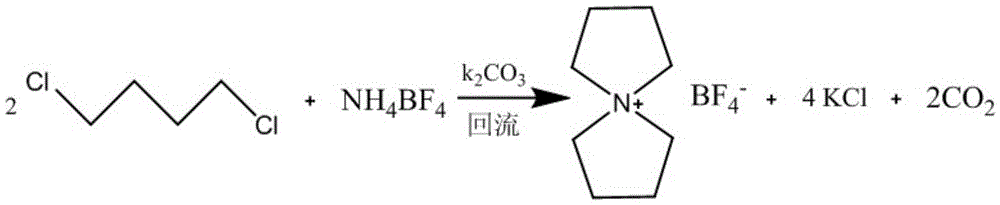
A kind of preparation method of symmetrical spirocyclic ammonium salt electrolyte
A spiro quaternary ammonium salt and electrolyte technology, which is applied in the directions of hybrid capacitor electrolytes, electrolytic capacitors, double-layer capacitors, etc., can solve the problems of strong acid safety hazards, long synthesis process, high energy consumption, and achieve high safety and good selectivity. , the effect of high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: Preparation of tetrafluoroborate spiro 1,1'-bispyrrole quaternary ammonium salt
[0027] In the three-necked flask, put 105g (1mol) NH 4 BF 4 , 254g (2mol) 1,4-dichlorobutane, 276g (2.1mol) K 2 CO 3 and 2000ml of deionized water, after reflux reaction at normal pressure for 10h, the resulting reaction solution was concentrated to a product content of 21%.
[0028] After cooling the concentrated solution, filter to remove the filter residue, and further concentrate the obtained filtrate to a paste solid with a product content of 75%, add an appropriate amount of ethanol, stir and crystallize at -10°C for 10 hours, and filter to obtain colorless transparent crystals.
[0029] The above crystals were dried under vacuum for 24 hours to obtain 184 g of spiro 1,1'-bispyrrole quaternary ammonium tetrafluoroborate in the form of colorless crystals. The molar yield is 86.3%.
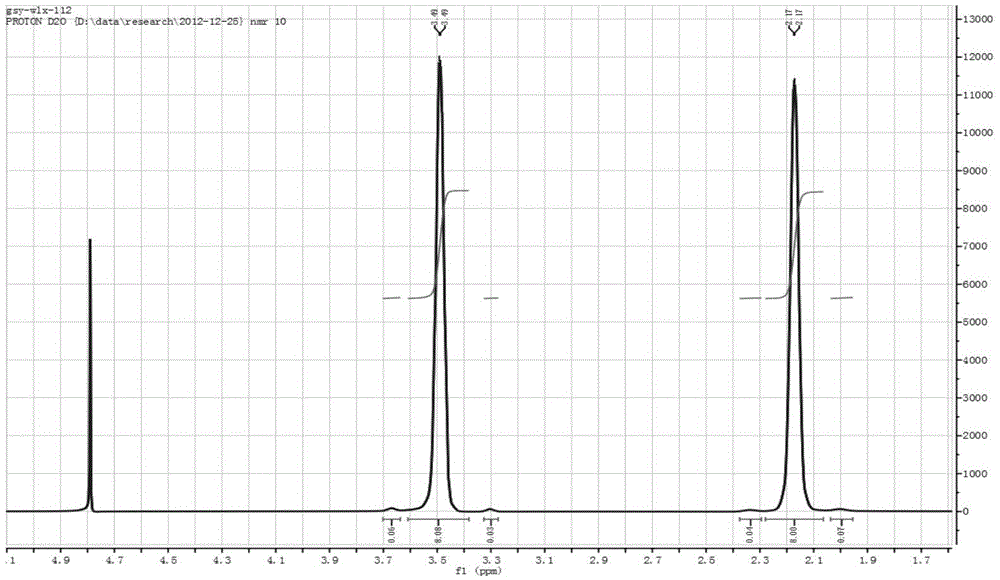
[0030] Proton NMR spectrum results: 1H NMR (400MHz, D 2 O): δ=2.17(8*H,4*CH 2 ), δ...
Embodiment 2
[0034] Embodiment 2: Preparation of spiro trifluoromethanesulfonate 1,1'-bispyrrole quaternary ammonium salt
[0035] In the three-necked flask, put 167g (1mol) NH 4 SO 3 CF 3 , 254g (2mol) 1,4-dichlorobutane, 212g (2mol) Na 2 CO 3 and 2000ml of deionized water, after reflux reaction at normal pressure for 18h, the resulting reaction solution was concentrated to a product content of 20%.
[0036] After cooling the concentrated solution, filter to remove the filter residue, and further concentrate the obtained filtrate to a paste solid with a product content of 70%, add an appropriate amount of isopropanol, stir and crystallize at -5°C for 10 h, and filter to obtain colorless transparent crystals.
[0037] The above crystals were dried under vacuum for 24 hours to obtain 224 g of colorless crystals of spiro 1,1'-bispyrrole quaternary ammonium trifluoromethanesulfonate, with a molar yield of 81.5%.
[0038] The product purity calculated by ion chromatography is 99.9%
[00...
Embodiment 3
[0041] Example 3: Preparation of difluorooxalate borate spiro 1,1'-bispyrrole quaternary ammonium salt
[0042] In the three-necked flask, put 155g (1mol) NH 4 B(C 2 o 4 )F 2 , 516g (2mol) 1,4-dibromobutane, 212gNa 2 CO 3 (2mol) and 2000ml of deionized water, reflux reaction at normal pressure for 20h, and then concentrate the resulting reaction solution to a product content of 19%.
[0043] After cooling the concentrated solution, filter to remove the filter residue, and further concentrate the obtained filtrate to a paste solid with a product content of 70%, add an appropriate amount of n-butanol, stir and crystallize at 0°C for 10 h, and filter to obtain colorless transparent crystals.
[0044] The above-mentioned crystals were dried under vacuum for 24 hours to obtain 240 g of colorless crystalline difluorooxalate borate spiro 1,1'-bispyrrole quaternary ammonium salt. The molar yield is 91.3%.
[0045] The product purity calculated by ion chromatography is 99.9%
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com