Iridoid ester compounds, and preparation method and application thereof
A technology of iridoid esters and compounds, which is applied in the field of active ingredients of natural medicines, and achieves the effects of low production cost, novel structure and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
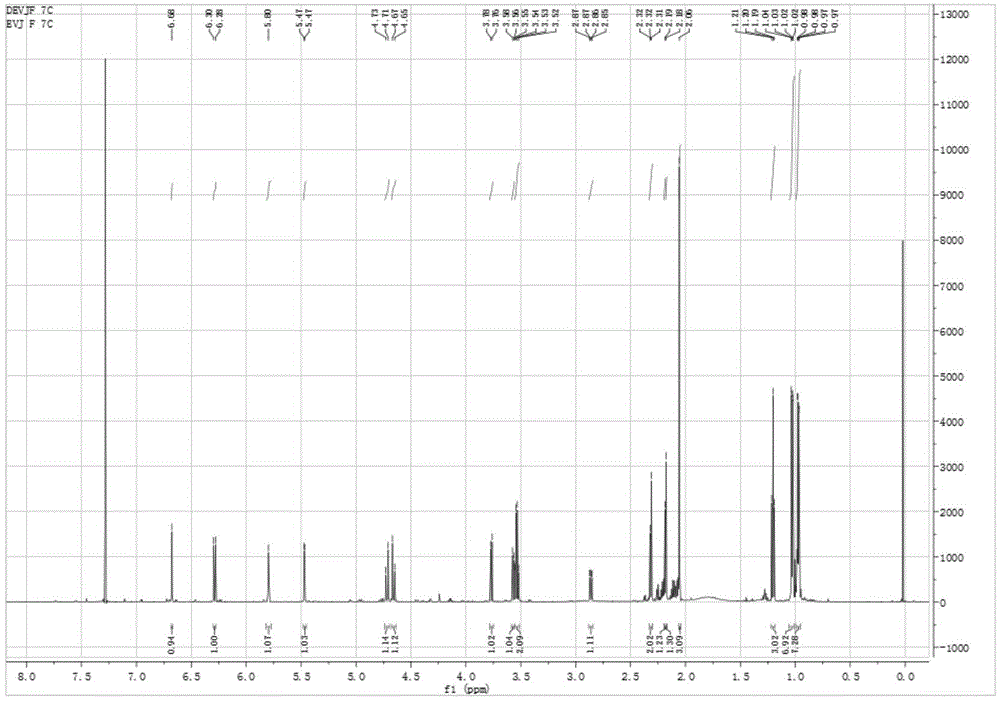
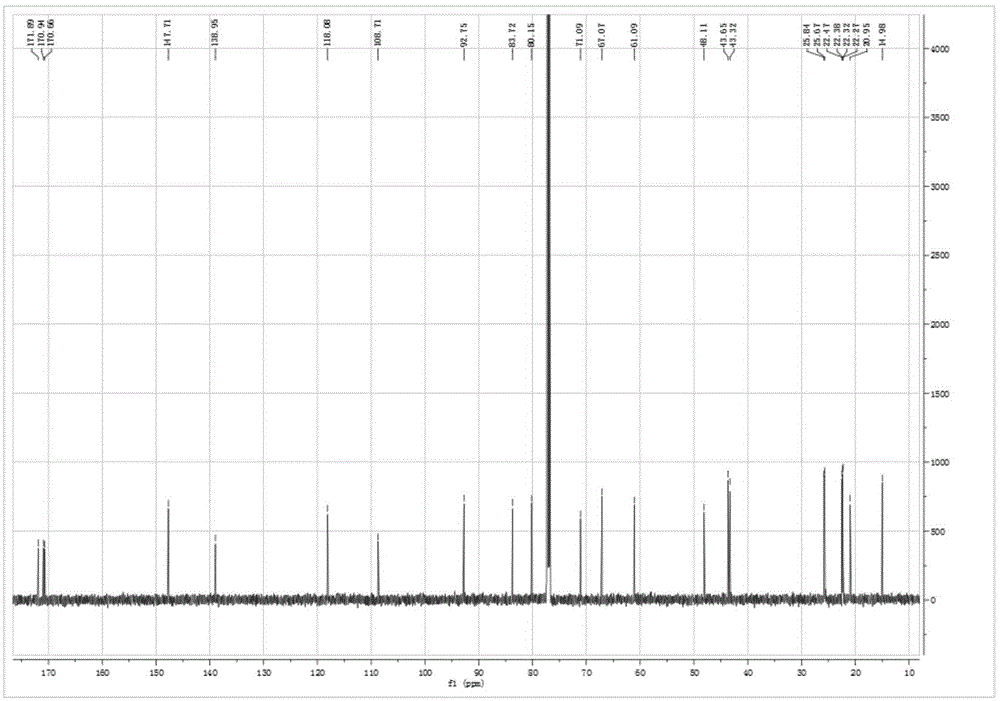
Embodiment 1
[0042] The preparation method of two novel iridoid esters in this valerian plant comprises the following steps:
[0043] A. Extraction: powder the medicinal materials of spider incense, and pass through a 40-mesh sieve for later use. Take 2kg of the coarse powder of spider incense, add an appropriate amount of extraction solvent to moisten it, then pack it into a column, add an appropriate amount of 95% ethanol for cold soaking for 24 hours, then extract by percolation, and collect the percolation liquid. The volume of the total solvent for extraction is equivalent to 15 times of the quality of the medicinal material (30 L of 95% ethanol is used in total).
[0044] B. Extraction: Concentrate the percolation liquid obtained in step A to nearly dryness under reduced pressure to obtain 148.4 g of thick extract, add water to suspend to 1 L, and use 1 L of dichloromethane, 1 L of ethyl acetate, and 1 L of n-butanol respectively Extraction, each extraction solvent was extracted thr...
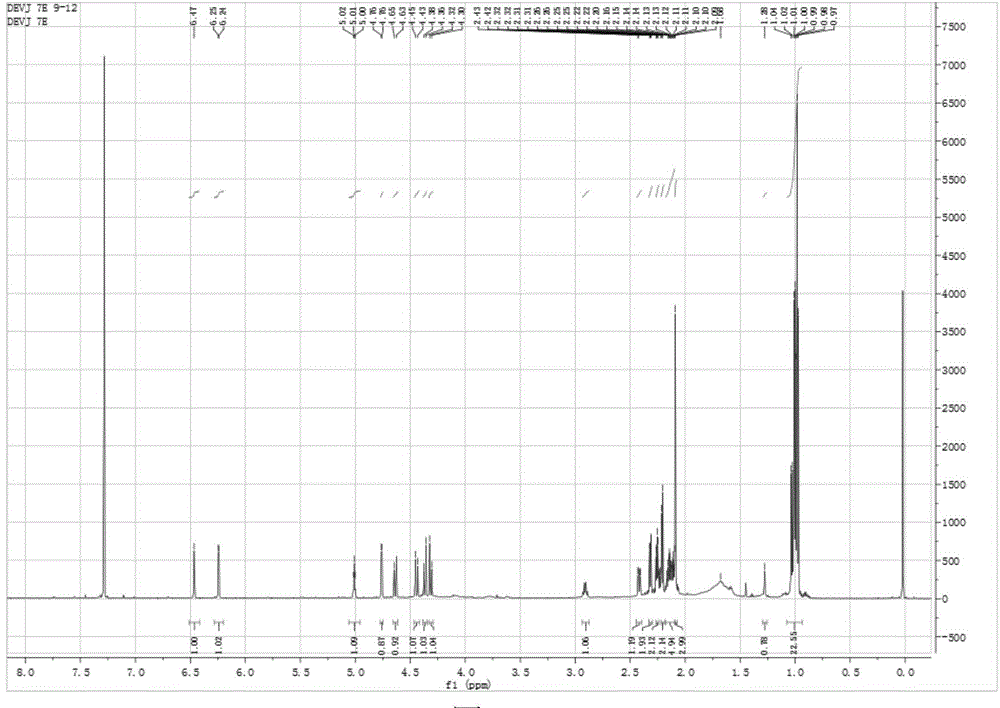
Embodiment 2
[0049] A. Extraction: take 2 kg of the medicinal material of C. chinensis, add 8 times the amount of 95% ethanol, and extract under reflux at 85°C. The extraction time is 2 hours each time. A total of 2 extractions are made, and the two extracts are combined. A total of 32L of 95% ethanol was used.
[0050] B. Extraction: Concentrate the extract obtained in step A to near dryness under reduced pressure to obtain 157.6 g of thick extract, add water to suspend to 1 L, extract with 1 L of dichloromethane and 1 L of ethyl acetate respectively, and extract with each extraction solvent Three times, the extracts of each time were combined to obtain the dichloromethane extract and the ethyl acetate extract respectively. Through TLC inspection, the iridoid esters of spider incense were mainly concentrated in the dichloromethane extract. The solvent was recovered from the methyl chloride extract under reduced pressure to obtain 97.3 g of the dichloromethane extract of the spider incense...
Embodiment 3
[0062] In vitro anti-tumor experiments of two novel iridoid ester compounds:
[0063] Take the human-derived tumor cell lines in the logarithmic growth phase, digest and count them, and divide them into 6~9×10 3 Cells / 100 μL / well were inoculated in a 96-well cell culture plate, cultured for 24 hours, and treated with different concentrations of compounds 1 and 2 prepared in Example 1 after the cells adhered to the wall, and three replicate wells were set in each well. After incubating the drug with the tumor cells for 48 hours, add MTT, continue to incubate at 37°C for 4 hours, stop the culture, gently absorb the culture solution with a pipette gun, add DMSO (150 μL / well), shake evenly, and use a microplate reader to The optical density OD value of each well was measured at 570nm, the OD value of each parallel well was averaged, and the background OD value was subtracted from the OD value of each test well. Calculate the inhibitory rate of drugs on tumor cells.
[0064] Inhi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com