Bone repair promoter
A bone repair and enhancer technology, applied in bone diseases, antibody medical components, anti-hormone immunoglobulin, etc., to achieve the effect of improving prognosis and reducing risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
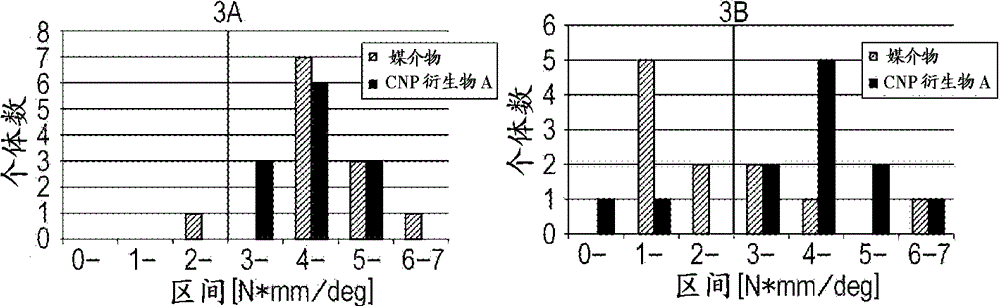
[0102] Example 1: Rat Femoral Fracture Model Test
[0103] [method]
[0104] The left femoral shaft of a 6-week-old female SD strain (Crl: CD) IGS rat was operated to prepare a fracture model. Barbituryl anesthetic (Somnopentyl®, Kyoritsu Seiyaku Corporation) was intraperitoneally administered to the animal to anesthetize the animal. Perform isoflurane (Escain, Mylan Inc.) inhalation anesthesia as an aid to anesthesia. Cut the skin on the upper side of the knee joint, and peel between the rectus femoris and lateral femoris muscles to expose the femur. At about 0.5 mm distal to the center of the femoral shaft, a bone cutter (Osada Equipment Co., Ltd.) was used to cut the shaft completely in cross section. Then, insert a 21G injection needle into the bone marrow between the left greater trochanter and femoral neck, and suture the muscle and skin with 2 to 3 sutures. In order to prevent postoperative suppuration, antibiotics penicillin G potassium for injection (Meiji Seika Kaisha...
Embodiment 2
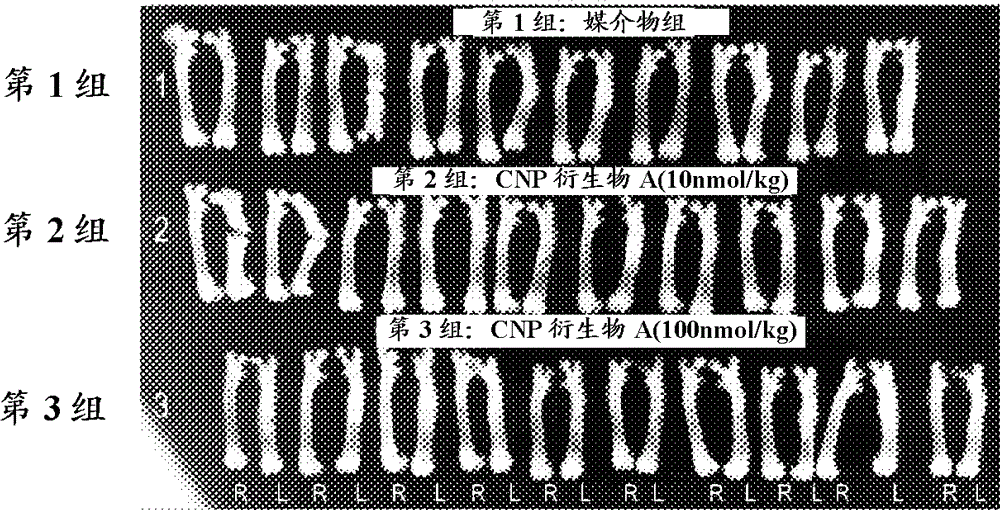
[0118] Example 2: Research on the promotion of fracture healing of CNP derivative A in rat closed femoral fracture model
[0119] [method]
[0120] 10-week-old male Wistar rats (Charles River Laboratories Japan, Inc.) were anesthetized by inhalation of isoflurane (5% by inhalation, 2% maintained) and kept in a supine position. Shave the body surface of the left knee with scissors and disinfect with chlorhexidine. Cut the skin of the left knee on the upper side of the patella about 2 cm. Cut the joint capsule together with the outer edge of the patella about 5 mm, and then dislocate the patella inward from the trochlear groove. Insert the 22G injection needle into the medullary cavity in the center of the trochlear groove and pull it out. The Kirschner wire (MIZUHO Corporation), which was previously cut to 32 mm and has a diameter of 1 mm, was inserted into the hole and completely inserted into the femoral medullary cavity. The joint capsule and the incision of the skin were sut...
Embodiment 3
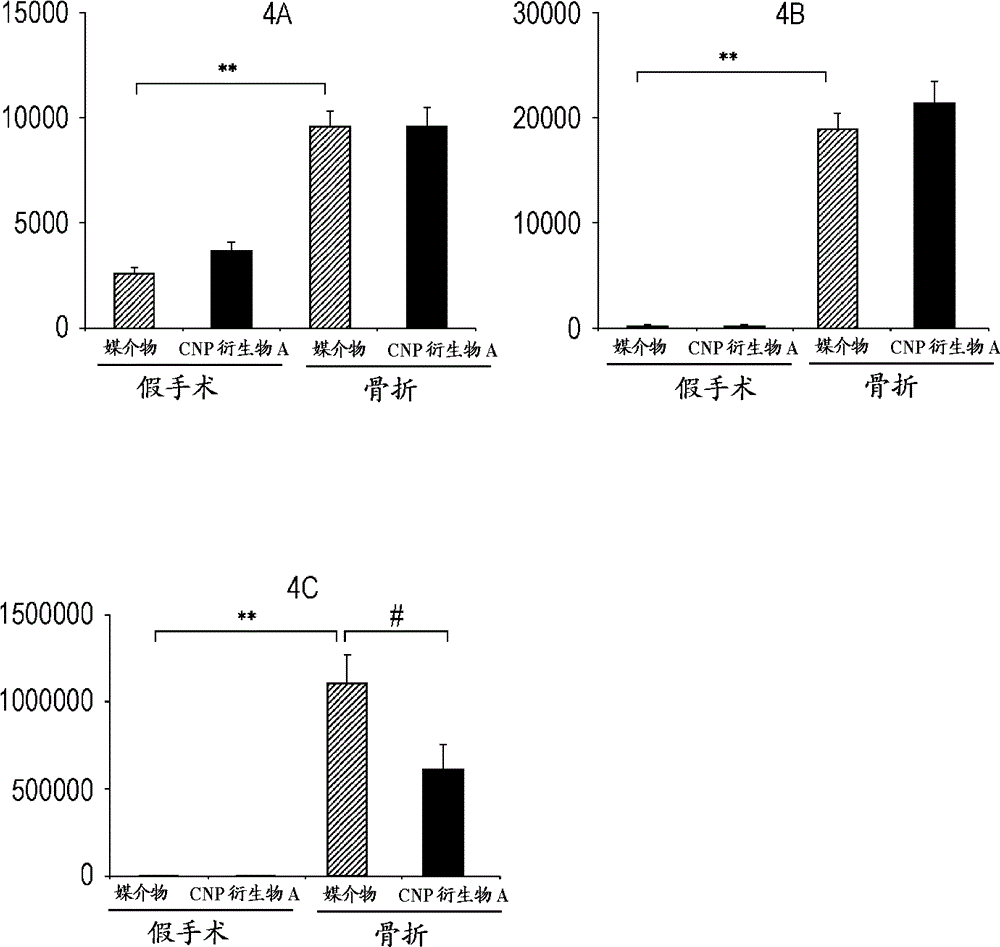
[0124] Example 3: The effect of CNP derivative A on the expression of cartilage-related marker genes in a rat closed femoral fracture model
[0125] [method]
[0126] According to the method of Example 2, a rat closed fracture model was prepared. For animals in the sham operation group, only the needle was inserted into the femur and no fracture was prepared. The administration solution of CNP derivative A was prepared to obtain a dose equivalent to 0.5 mg / kg / day, and then filled into an osmotic pump (Model 2001, Alzet). The osmotic pump was buried under the skin behind the animal's neck and administered subcutaneously for 1 week. The vehicle was continuously subcutaneously administered to the control group. Seven days after the preparation of the fracture, the animals were subjected to isoflurane inhalation anesthesia, maintained in a supine position to open the abdomen, and then euthanized by bleeding. The left femur was excised and immersed in RNAlater (Life Technologies Cor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com