Icariin controlled-release chitosan/hydroxyapatite composite scaffold material and preparation method thereof
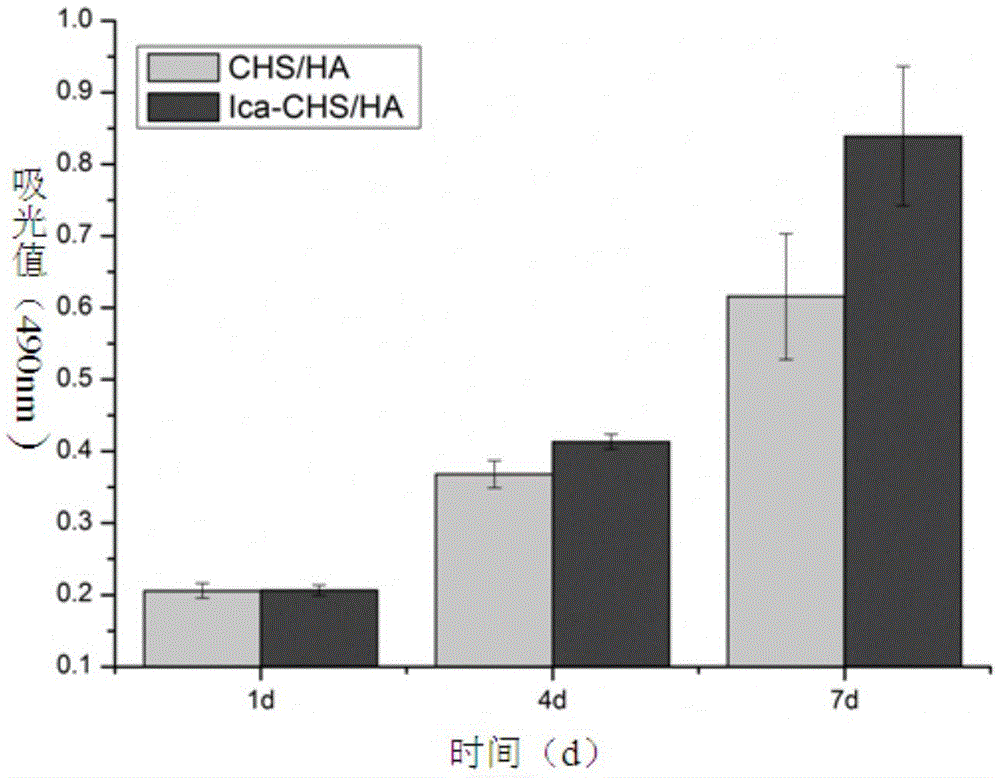
A technology of hydroxyapatite and icariin, which is applied in the field of biomedical materials, can solve the problems of limited loading and achieve the effects of improving bone repair performance, facilitating cell proliferation, increasing expression and calcium deposition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] (1) Preparation of raw material solution
[0055] Using chitosan, hydroxyapatite and succinylated icariin as raw materials, the mass ratio of chitosan to hydroxyapatite is 1:9, and the raw material solution is prepared according to the following method:
[0056] Under normal pressure and room temperature, use 0.5% dilute hydrochloric acid as solvent to prepare chitosan solution, and the consumption of hydrochloric acid reaches 30mg / ml as the limit with the concentration of chitosan in chitosan solution;
[0057] Hydroxyapatite was prepared into a 27% w / v slurry with deionized water at normal pressure and room temperature;
[0058] Under normal pressure and room temperature, the succinylated icariin was formulated with 0.2mol / L sodium hydroxide to make 1×10 -3 mol / L solution;
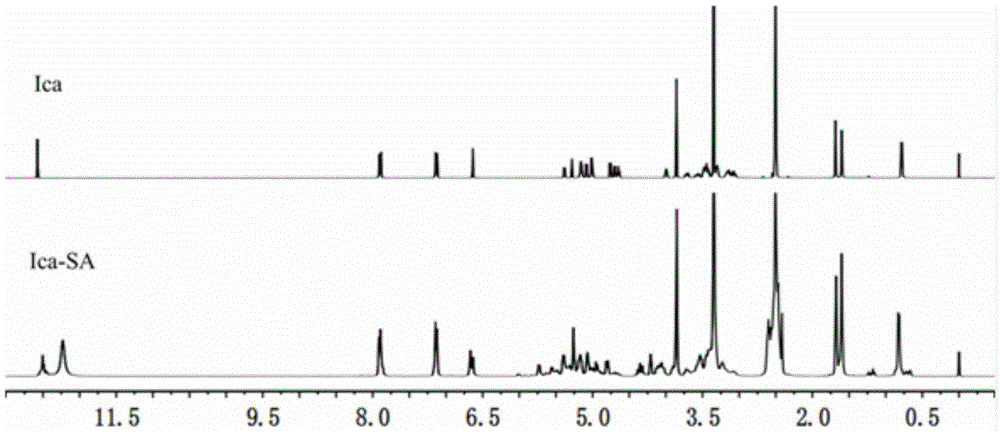
[0059] The structural formula of the succinylated icariin is:
[0060]
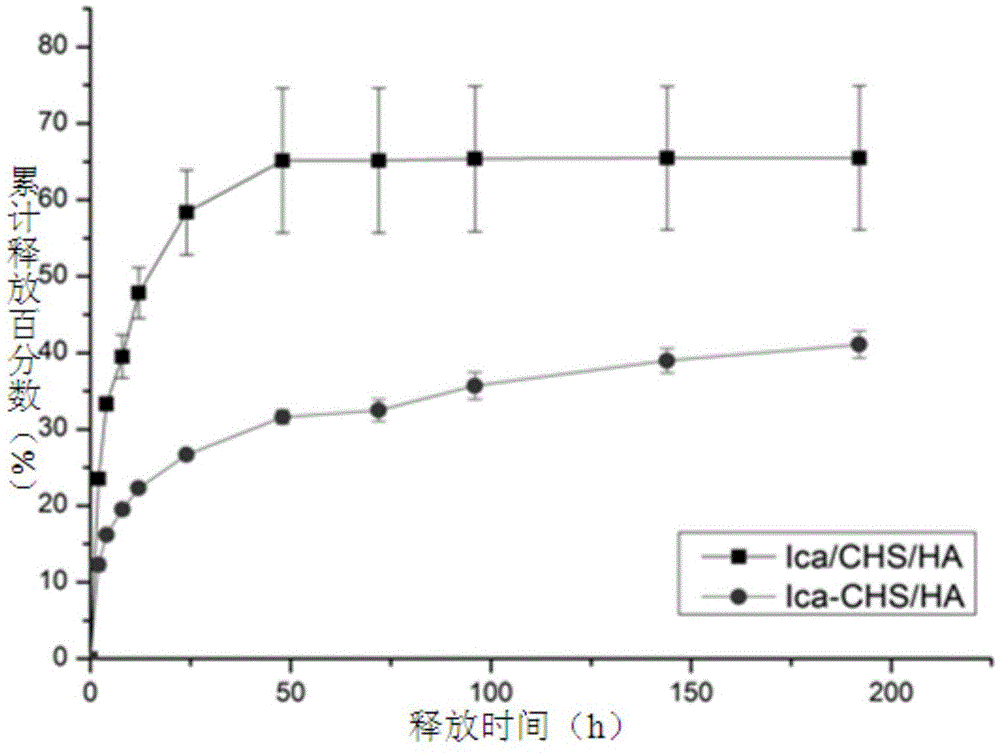
[0061] (2) Preparation of icariin controlled-release chitosan / hydroxyapatite composite scaffold material
[0062] St...
Embodiment 2
[0065] (1) Preparation of raw material solution
[0066] Using chitosan, hydroxyapatite and succinylated icariin as raw materials, the mass ratio of chitosan to hydroxyapatite is 1:1, and the raw material solution is prepared according to the following method:
[0067] Under normal pressure and room temperature, the hydrochloric acid with the volume concentration of 0.7% is the solvent preparation chitosan solution, and the consumption of hydrochloric acid reaches 60mg / mL as the limit with the concentration of chitosan in the chitosan solution;
[0068] Hydroxyapatite is prepared into a 12% w / v slurry with deionized water or distilled water at normal pressure and room temperature;
[0069] At normal pressure and room temperature, the succinylated icariin was formulated with a 0.2mol / L sodium hydroxide solution to a concentration of 1×10 -1 mol / L solution;
[0070] (2) Preparation of icariin controlled-release chitosan / hydroxyapatite composite scaffold material
[0071] The ...
Embodiment 3
[0074] (1) Preparation of raw material solution
[0075] With chitosan, hydroxyapatite and succinylated icariin as raw materials, the mass ratio of chitosan to hydroxyapatite is 2:3, and the raw material solution is prepared according to the following method:
[0076] Under normal pressure and room temperature, the hydrochloric acid with the volume concentration of 1% is the solvent preparation chitosan solution, and the consumption of hydrochloric acid reaches 40mg / mL as the limit with the concentration of chitosan in the chitosan solution;
[0077] Hydroxyapatite was prepared into a 6% w / v slurry with deionized water at normal pressure and room temperature;
[0078] At normal pressure and room temperature, the succinylated icariin was formulated with a 0.5mol / L sodium hydroxide solution to a concentration of 1×10 -1 mol / L solution;
[0079] (2) Preparation of icariin controlled-release chitosan / hydroxyapatite composite scaffold material
[0080] Stir and mix the chitosan ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| degree of deacetylation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com