Method for preparing self-cleaning type surface raman-enhanced substrate
A surface Raman-enhanced and self-cleaning technology, used in Raman scattering, chemical instruments and methods, liquid chemical plating, etc., can solve the problems of great influence on the performance of nanomaterials, and achieve self-cleaning and excellent photocatalytic degradation. The effect of performance and preparation process is simple
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
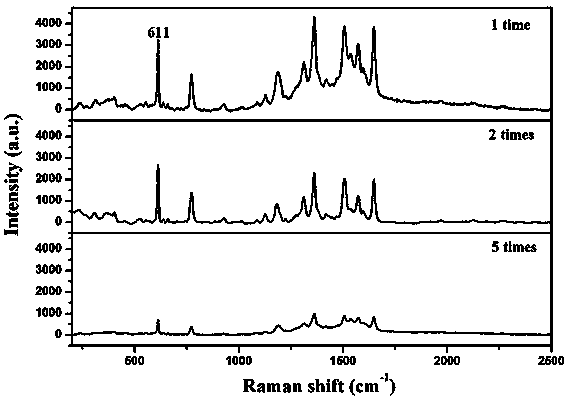
[0027] Example 1 mainly investigates the effect of the amount of PDDA on the structure and morphology of the AgAgCl complex.
[0028] Add 150mg-1000mg PDDA dropwise to 20 mL silver nitrate aqueous solution (0.02 mol / L), and stir magnetically for 10 min at room temperature to obtain Ag + / PDDA suspension.
[0029] Add 20 mL NaCl aqueous solution (0.02 mol / L) and 60 mL C 6 h 12 o 6 The aqueous solution (0.84mmol / L) was added to the above four suspensions respectively, and the milky white AgCl sol was obtained after magnetic stirring for 60 min.
[0030] Finally, 100 mL of the above colloidal solution was transferred into an autoclave, kept at 180 °C for 24 h, cooled naturally, and finally centrifuged and washed three times with water, twice with alcohol, and once with acetone, and then dissolved in 20 mL of water. Obtain AgAgCl sol.
[0031] figure 1 (a-c) are the SEM images of AgAgCl sols prepared with 150 mg, 500 mg and 1000 mg of PDDA, respectively. It can be seen from...
Embodiment 2
[0033] Example 2 mainly investigates the effect of hydrothermal reaction time on the structure and morphology of AgAgCl composite powder.
[0034] Add 500 mg of PDDA dropwise to 20 mL of silver nitrate aqueous solution (0.02 mol / L), and magnetically stir at room temperature for 10 min to obtain Ag + / PDDA suspension.
[0035] Add 20 mL NaCl aqueous solution (0.02 mol / L) and 60 mL C 6 h 12 o 6 Aqueous solution (0.84 mmol / L) was added to the above suspension, and a milky white AgCl sol was obtained after magnetic stirring for 60 min.
[0036]Finally, transfer 100 mL of the above colloidal solution into an autoclave, under hydrothermal conditions, hydrothermally react at 180 °C for 12 h, 24 h, and 36 h, then cool naturally, and finally centrifuge and wash 3 times with water, wash with alcohol twice, and wash with acetone 1 time, and then dissolved in 20 mL of water to obtain three groups of AgAgCl sols.
[0037] figure 2 (a-c) are SEM pictures of hydrothermal reaction tim...
Embodiment 3
[0040] First prepare GO into a suspended aqueous solution with a concentration of 0.1 mg / mL and ultrasonically disperse until uniformly dispersed, then mix 10 mL of the AgAgCl sol prepared by hydroheating for 24 h in Example 2 with 10 mL of GO aqueous solution, and ultrasonically disperse for 2 h before processing Soak the glass substrate in the above mixed solution, take it out after 30min, blow dry and soak again, repeat the above steps 4 times in this way, and make the AgAgCl / GO composite thin film.
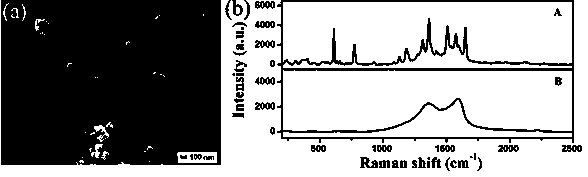
[0041] image 3 (a) is the SEM image of the AgAgCl / GO composite film; we know that the oxygen-containing groups on the GO sheet are negatively charged, and the AgAgCl sol prepared by the hydrothermal reaction is positively charged due to the addition of PDDA, thus ensuring that the two can Composite and deposited on the glass substrate by electrostatic self-assembly, the obvious film-like wrinkled morphology of GO can be clearly seen from the figure, the AgAgCl nanoparticles a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com