Method for increasing cell phloroglucinol synthesis yield and application
A phloroglucinol and cell technology, applied in the field of genetic engineering, can solve the problems of unknown metabolism, phloroglucinol production and yield, etc., and achieve the effect of improving capacity and reducing production and emission
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Gene multiple resistance factor gene MarA (GenBank accession number: 6060688), polyketene anhydride synthase gene PhlD (GenBank accession number: EU554263), fructose 1,6-bisphosphatase gene fbp (GenBank: ACT45889.1), And the phosphoketolase gene fxpk (GenBank: AY518212.1) was linked together by the method of overlap extension PCR, and then cloned on pET30a by homologous recombination to construct the recombinant plasmid pET-phlD-marA-fxpk-fbp. At the same time, construct the plasmid pA-accADBC, the specific method is the same as the patent CN 102787135B.
Embodiment 2
[0032] The recombinant plasmids pET-phlD-marA-fxpk-fbp and pA-accADBC constructed in Example 1 were co-transformed into Escherichia coli BL21 (DE3) by the heat shock transformation method, and then spread on the culture medium with kanamycin and chloramphenicol Positive clones were screened on LB solid medium plates resistant to two types of protein to obtain recombinant Escherichia coli LWNOGPG1. The control bacteria were constructed by co-transforming Escherichia coli BL21(DE3) with the recombinant plasmids pET-phlD-marA and pA-accADBC by the heat shock transformation method to obtain recombinant Escherichia coli LWNOGPGO (all examples use this as the control bacteria).
Embodiment 3
[0034] The recombinant Escherichia coli fermented and produced in Example 2 is used to produce phloroglucinol, and the steps are as follows:
[0035] Inoculate the recombinant cells at an inoculum volume of 1% by volume to add 50 μg·mL -1 Kanamycin and 30 μg·mL -1 Fermentation was carried out in the M9 fermentation medium of chloramphenicol, and cultured to OD under the conditions of culture temperature 30°C, stirring speed 400rpm, pH 6.0 600 8, add the inducer IPTG to a final concentration of 0.1mmol L -1 , close the ventilation, and continue feeding 40% by weight of glucose to ferment for 12 hours;
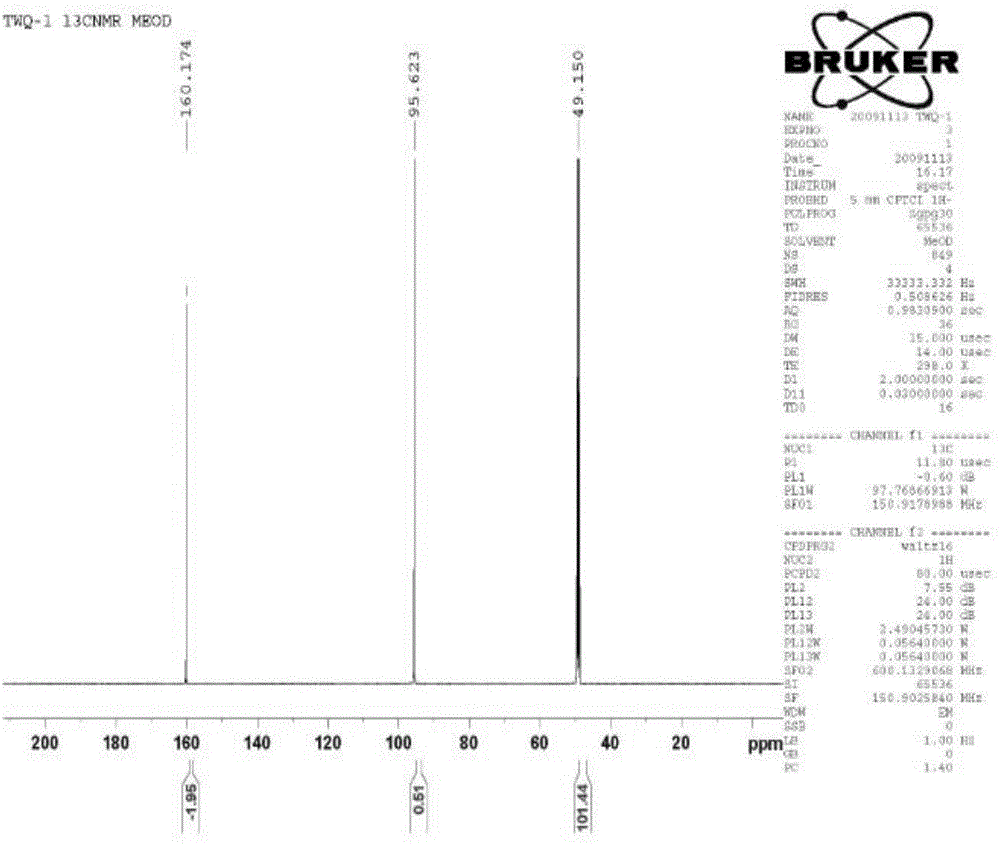
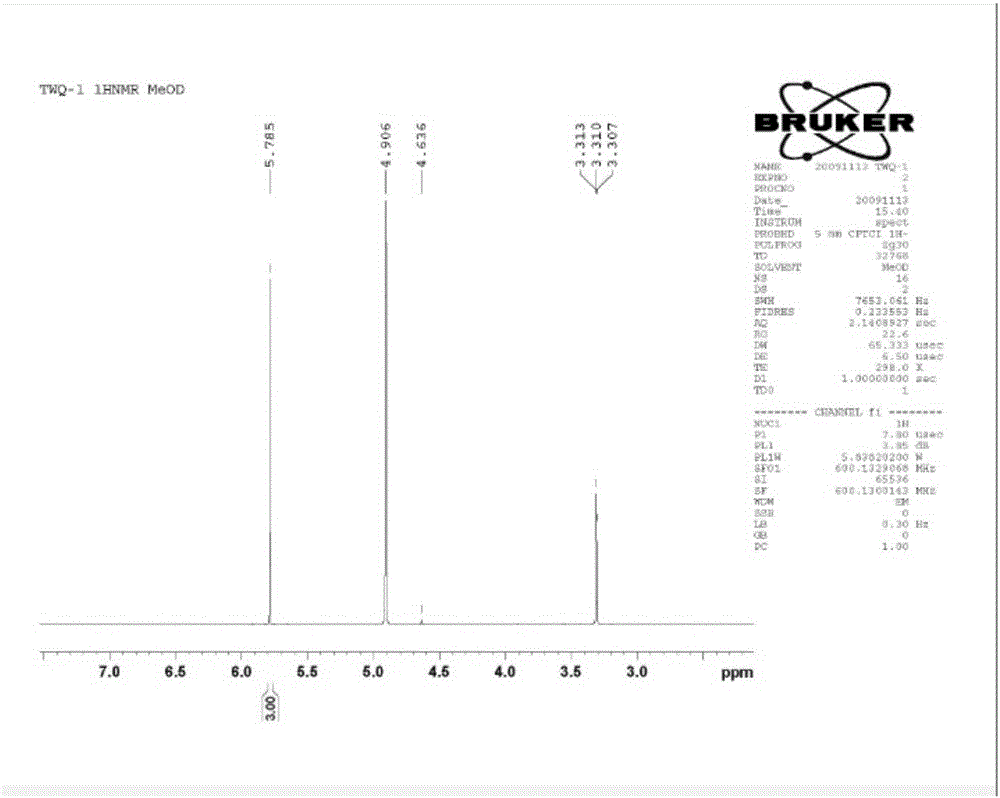
[0036] The culture medium was centrifuged to separate the cells and the supernatant, and the supernatant was extracted once with an equal volume of ethyl acetate; the extracted products were combined and concentrated by distillation under reduced pressure. figure 1 and figure 2 ) was identified as phloroglucinol, and the output per liter of culture medium reached 6.5 grams,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com