Laccase gene derived from laccaria bicolor, and applications thereof
A laccase and sequence technology, applied in the field of microorganisms, to achieve the effect of wide application potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Secretory expression of LbLAC3I gene in Pichia pastoris
[0024] 1. Construction of Pichia pastoris expression vector
[0025] Based on the wild-type gene sequence, the gene sequence of 23 amino acids constituting the N-terminal signal peptide was deleted. According to the preferred codons of Pichia pastoris, avoid PolyA tailing signals such as ATTTA in the gene, avoid 6 or more continuous A+T sequences, avoid 5 or more G+C sequences, and the ratio of G+C 40-60%, prevent intron cutting sequences, reduce the two-level structure hairpins inside the gene and optimize the gene, and eliminate the internal enzyme cutting site. The sequence is shown as SEQ ID NO 1, and the whole gene was synthesized by Nanjing GenScript Company.
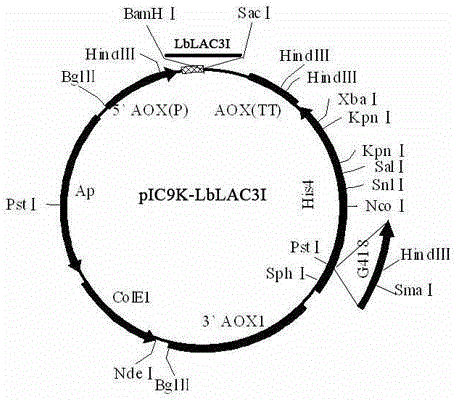
[0026] The gene fragment was ligated with T / A cloning vector (Takara). Transform DH5α competent to obtain positive clones. The positive clone plasmid was extracted, and the Bam H1 and Sac 1 After double digestion, insert the correct reading f...
Embodiment 2
[0034] Determination of the properties of LbLAC3I laccase: the analysis process was carried out in the following enzyme reaction system: 10 μL 1 mM ABTS, 20 μL 40 mM copper sulfate and 120 μL disodium hydrogen phosphate-citrate buffer buffer. The reaction was started by adding 50 μL of enzyme solution, and the reaction was carried out in a 37°C enzyme cutter for 30 min, and the reaction was terminated by adding 50 μL of 1 M NaF. Then measure the amount of oxidation to ABTS at 420 nm. In the present invention, the activity of a certain number of enzymes catalyzing 1 μmol of ABTS per minute is defined as 1U, and the protein concentration is analyzed using a Bradford kit.
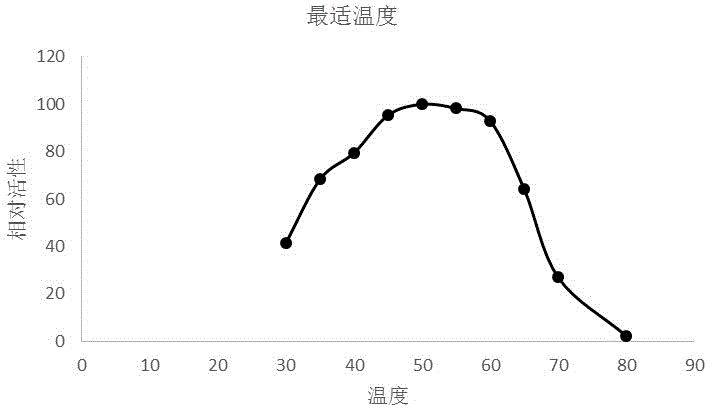
[0035] Optimum reaction temperature and temperature stability: Enzyme-catalyzed reaction is carried out at the optimum pH and different temperatures (20-70°C), and the optimum reaction temperature of LbLAC3I laccase is measured to be 40°C (see image 3 ). Treat the enzyme solution at different temperatures fo...
Embodiment 3
[0038] The decolorization effect of LbLAC3I laccase on the dye: add 10 μL 1mM malachite green to the reaction system (10 μL 1mM ABTS, 20 μL 40mM copper sulfate and 110 μL disodium hydrogen phosphate-citrate buffer buffer). The reaction was started by adding 50 μL of enzyme solution, and the reaction was carried out in a 37°C enzyme cutter for 4 h. Comparing the absorbance changes of malachite green at a wavelength of 620 nm before and after the reaction, the laccase has a significant decolorization effect. The detection method of crystal violet and orange yellow G is the same as that of malachite green, the detection wavelengths are 580 nm and 480 nm respectively, which can significantly decolorize (see Figure 7 ).
[0039]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com