Synthesis method of spiropyrane small-molecule fluorescent probe with extreme acid/extreme alkaline switch response and application of spiropyrane small-molecule fluorescent probe
A technology of fluorescent probes and spiropyrans, applied in the field of molecular fluorescent probes, to achieve the effects of fast pH response, excellent biocompatibility, and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
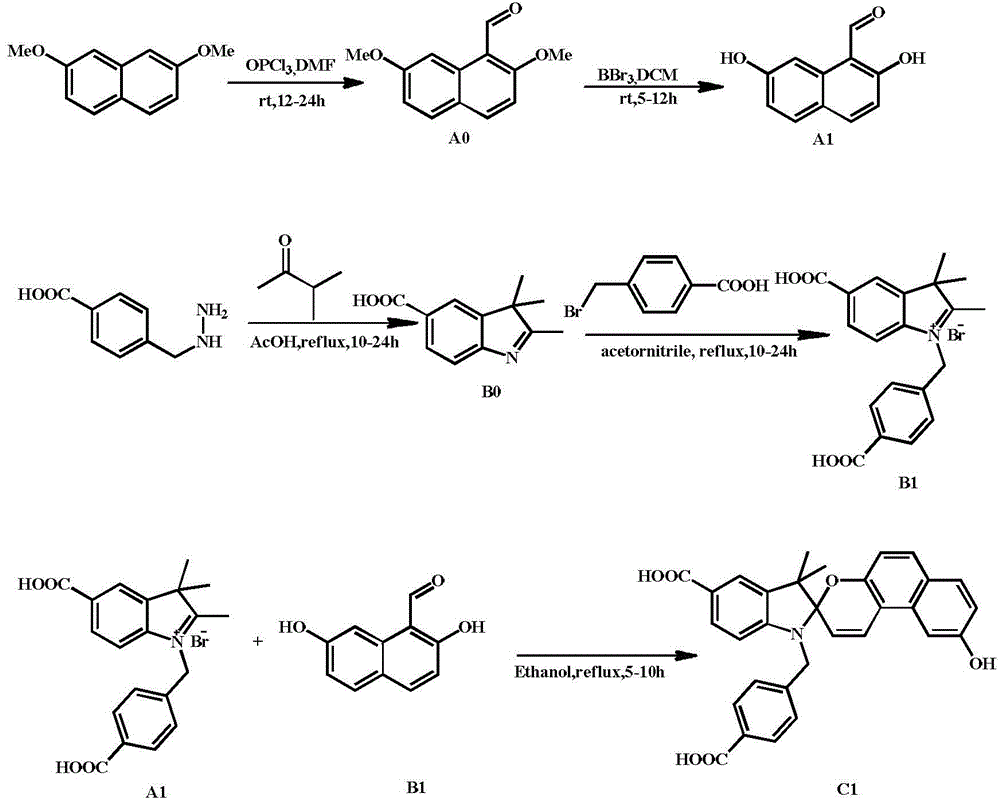
[0029] 1) Slowly add 43.3mmol of phosphorus oxychloride dropwise to 1.5mL of dimethylformamide in an ice bath, remove the ice bath, stir at room temperature for 30 minutes under a nitrogen atmosphere, then drop in 10mL of 2,7-dimethoxy The dichloromethane solution of dimethoxynaphthalene, in which 2,7-dimethoxynaphthalene is 5.3mmol, reacted at room temperature for 10h; after the reaction was completed, the reaction liquid was added dropwise into the ice-water mixture, hydrolyzed for 3h, extracted with dichloromethane, and then The solvent dichloromethane was evaporated to dryness, and finally recrystallized with methanol to obtain white needle-like crystals of 2,7-dimethoxy-1-aldehyde naphthalene, denoted as AO, with a yield of 78%;
[0030] 1 H NMR (CDCl 3,400MHz,ppm):δ10.90(s,1H,CHO),8.85(d,1H,ArH),7.98(d,1H,ArH),7.66(d,1H,ArH),7.12(d,1H, ArH),7.07(dd,1H,ArH),4.08(s,3H,OCH 3 ),3.98(s,3H,OCH 3 ). 13 C NMR (101MHz, CDCl 3 ): δ192.07, 164.86, 161.52, 137.37, 133.52, 129....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com