Bispecific antibody
A bispecific antibody and specific technology, applied in the direction of antibodies, specific peptides, anti-tumor drugs, etc., can solve problems such as unsuitable for cancer, limited effect, and prone to drug resistance in patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0249] Preparation method of couplet
[0250] Bispecific antibodies can be modified with appropriate bifunctional group modifying reagents. In some embodiments, a sulfhydryl (SH)-containing group can be introduced into the side chain of an amino acid residue, such as a lysine side chain. For example, the amino group of a lysine residue can be combined with 2-iminothiolane (Traut's Reagent) or with N-hydroxysuccinimide 3-(2-pyridinedithio)propionate (SPDP), 4-( Reactions such as N-hydroxysuccinimidyl 2-pyridinedithio)butyrate (DPDB) followed by reducing agents such as 2-mercaptoethanol, dithiothreitol (DTT) or tris(2-carboxyethyl)phosphine (TCEP) reductive conversion into thiol-containing groups.
[0251] Non-limiting examples of sulfhydryl-containing groups that can replace side chain amino groups on lysine residues include -NHC(=NH)(CH 2 ) n SH and -NHC(O)(CH 3 ) n SH, where n is 1, 2, 3, 4, 5 or 6. When a thiol-containing group is introduced into an amino acid residue...
Embodiment 1
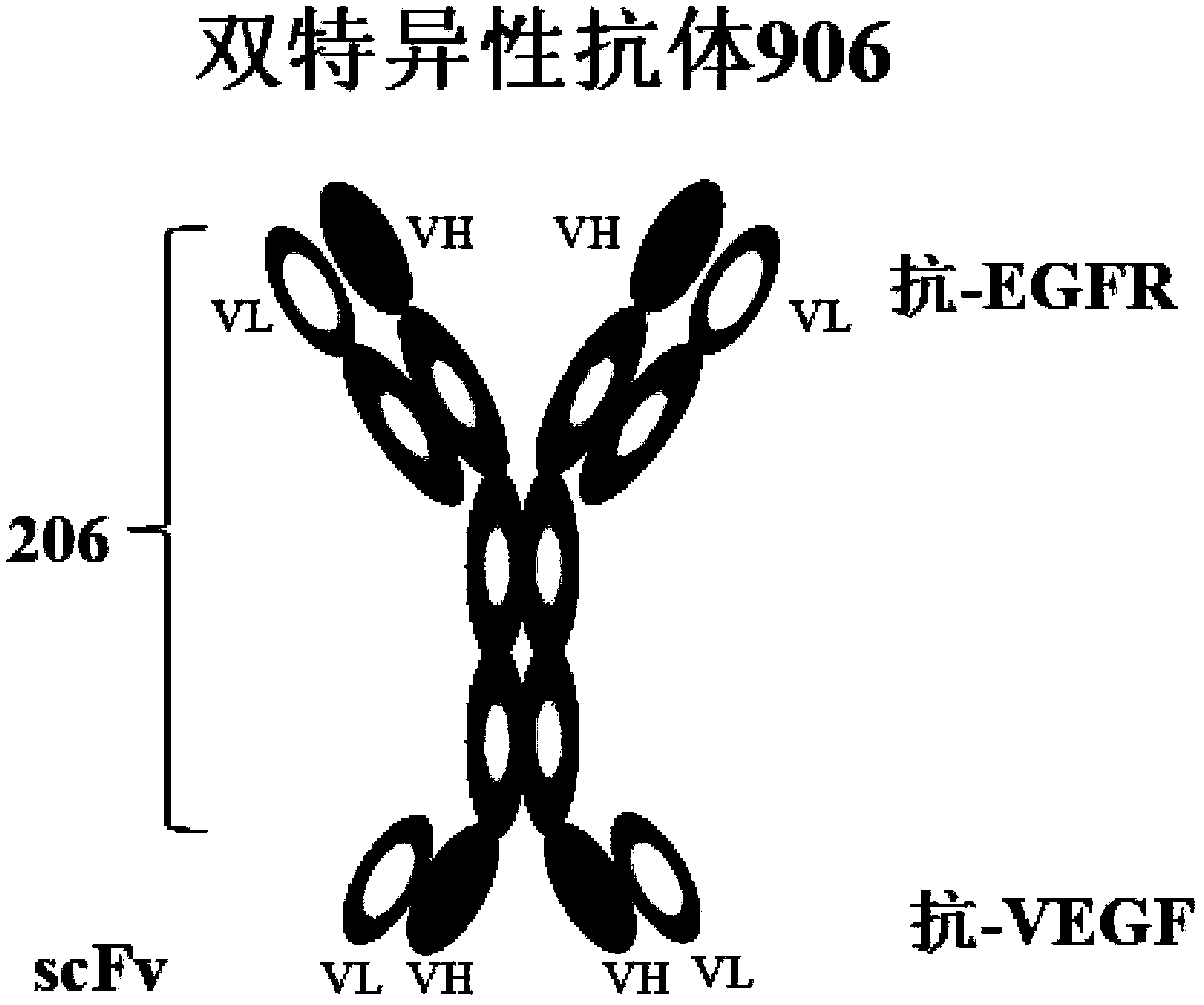
[0270] Construction and expression of recombinant anti-EGFR and anti-VEGF bispecific antibody (Antibody 906)
[0271] The host cell line used for antibody expression is a derivative cell line of CHO-K1 cells, grown in suspension in CD-CHO medium. The construction process of the stable cell line of the recombinant bispecific antibody (referred to as "Antibody 906") is as follows: First, the expression vector containing the antibody gene is constructed by conventional molecular biology methods, and the anti-VEGF single-chain antibody scFv is designed in the anti-EGFR The heavy chain C-terminus of the antibody (referred to as "Antibody 206"). The structure of the bispecific antibody is as Figure 1A shown in . The amino acid sequences of these chains are shown in Table 1 and Table 2. Then the constructed plasmid was digested and linearized.
[0272] Centrifuge host cells in logarithmic growth phase and resuspend in fresh CD-CHO medium (cell density 1.43×10 7 pieces / ml). Take...
Embodiment 2
[0275] Expression and purification of recombinant bispecific antibody The antibody purification process is briefly described as follows: After the cells are cultured on a large scale for 2 weeks, the cells and the medium are separated by low-speed centrifugation, and the harvested supernatant is further centrifuged at high speed to obtain a clarified feed solution. The recombinant antibody was purified by two-step affinity chromatography (Protein A) and ion exchange. The media used in the purification were Mab Select SuRe and Capto S produced by GE. The isolated and purified antibodies were analyzed by SDS-PAGE ( Figure 2B ) and molecular sieve chromatography identification (Fig. 2C). SDS-PAGE results showed that 906 bands of bispecific antibody molecules were monospecific, while 2016 bands of control bispecific antibody molecules were diffuse and multimerized. The results of molecular sieves further confirmed that the 906 antibody has almost no multimers. All these results...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com