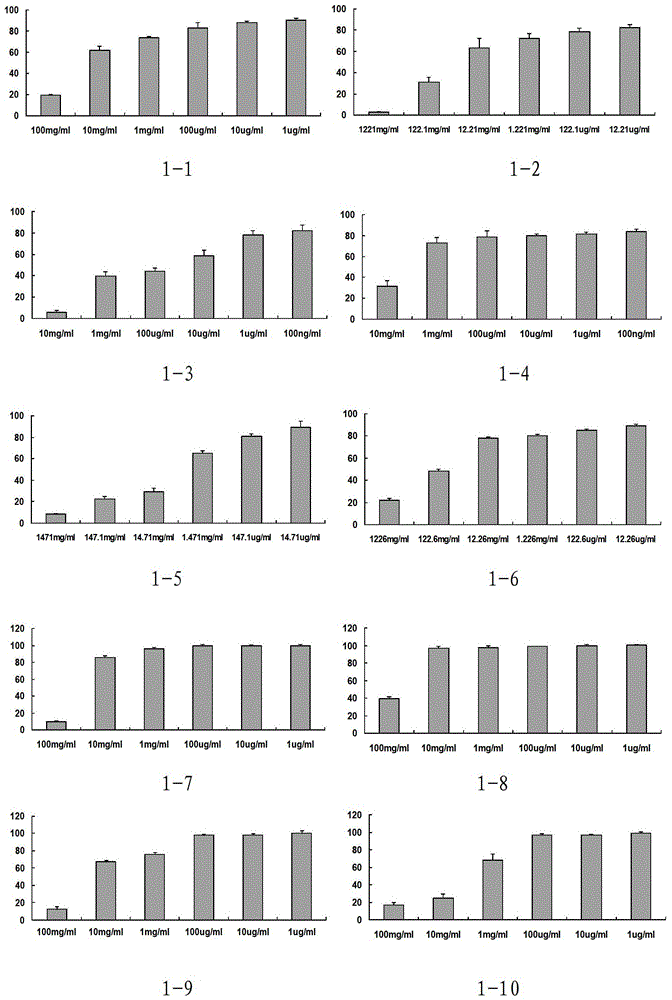
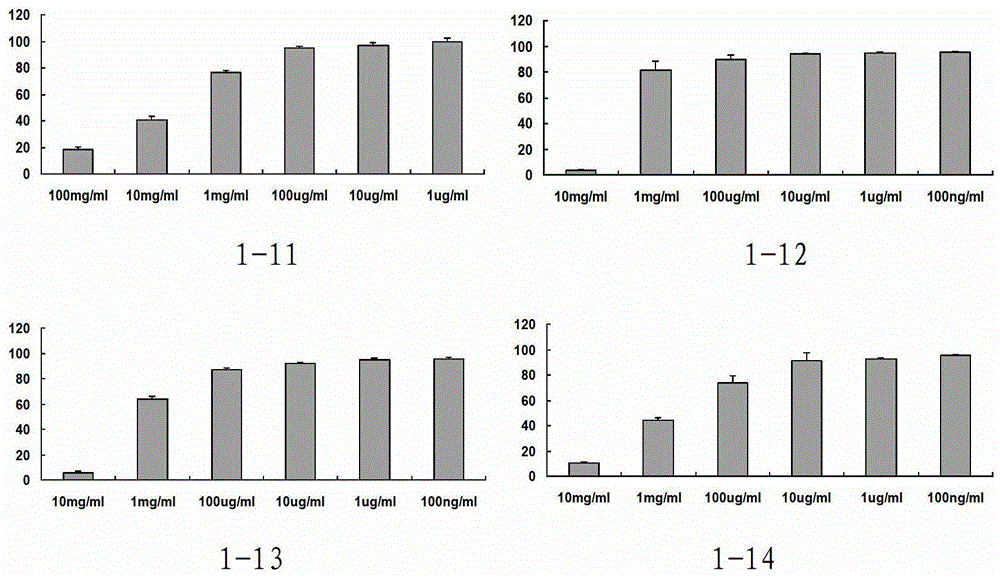
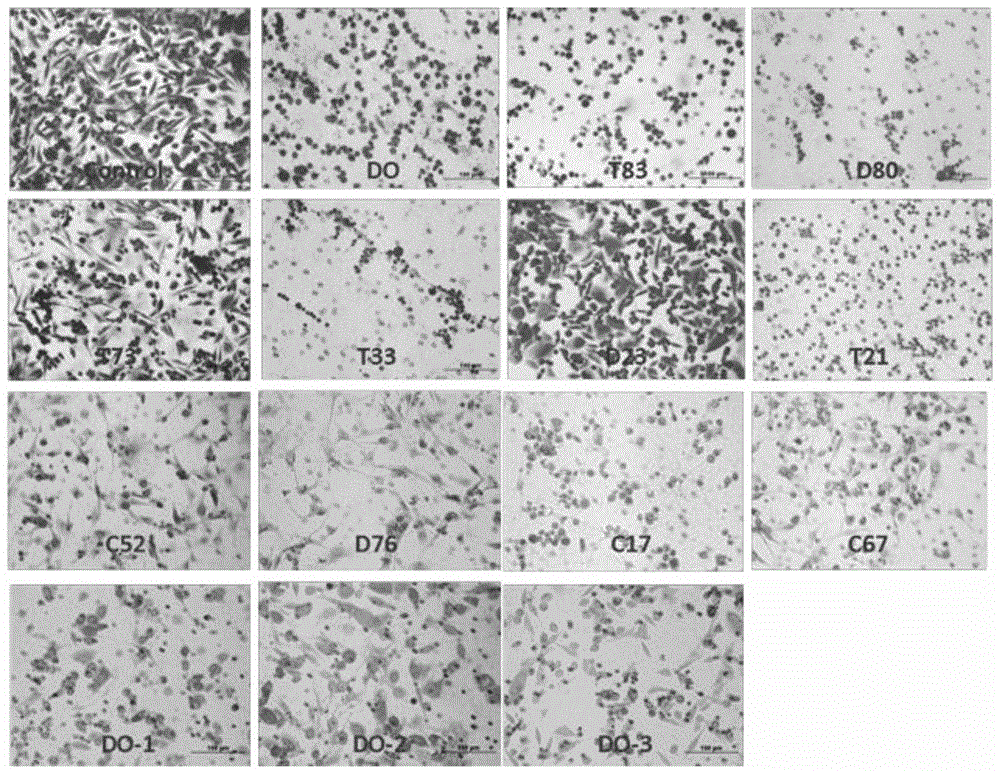
Phenylacetic acid derivatives and their antitumor applications
A use, tumor technology, applied in the field of pharmacy, can solve problems such as reproductive system damage, liver and kidney function damage, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0113] Example 1 Synthesis of methyl 3-hydroxy-4-methoxyphenylacetate (DO-2)
[0114]
[0115] 3-Hydroxy-4-methoxy-phenylacetic acid (DO-1, 2.5g, 13.7mmol) was dissolved in 50mL of methanol, 0.2mL of concentrated sulfuric acid was added under stirring conditions, and heated to reflux for 3 hours. Cool to room temperature, concentrate the reaction solution on a rotary evaporator, dilute the obtained oil into 100 mL of ethyl acetate solution, wash the organic phase with saturated brine (50 mL*2), dry and filter with anhydrous sodium sulfate, and concentrate by rotary The solvent was removed, and the solute was purified by column chromatography (mobile phase: petroleum ether: ethyl acetate = 3:1) to obtain DO-2 (2.1g, 79%) as a colorless oil.
[0116] 1H-NMR (400MHz, CDCl 3 )δ6.80 (s, 1H), 6.75-6.68 (m, 2H), 5.56 (s, 1H), 3.81 (s, 3H), 3.62 (s, 3H), 3.31 (s, 3H).
Embodiment 2
[0117] Example 2 Synthesis of Ethoxymethyl 3,4-Dihydroxyphenylacetate (DO-3)
[0118]
[0119] 3,4-Dihydroxyphenylacetic acid (DO, 5.0g, 30.0mmol) and triethylamine (9.1g, 90.0mmol) were dissolved in 30mL of dichloromethane, and 2.84g of chloromethyl ethyl ether (30.0mmol ), and the reaction mixture was stirred overnight at room temperature. The reaction solution was mixed with 0.1N HCl solution (15mL) and NaHCO 3 solution (15mL), the organic phase was dried and filtered with anhydrous sodium sulfate, the solvent was removed by rotary concentration, and the solute was purified by column chromatography (mobile phase: petroleum ether: ethyl acetate = 2:1) to obtain a colorless oil DO- 3 (2.0g, 29.5%).
[0120] 1 H-NMR (400MHz, CDCl 3 )δ6.72(d,J=2.0Hz,1H),6.69(s,1H),6.62(m,1H),5.65(brs,1H),5.45(brs,1H),5.24(s,2H), 3.60(q,J=6.4Hz,2H),3.48(s,2H),1.14(t,J=6.4Hz,H).
Embodiment 3
[0121] Example 3 Synthesis of ethyl 3,4-dihydroxyphenylacetate (D80)
[0122]
[0123] 3,4-Dihydroxyphenylacetic acid (DO, 3.0g, 17.9mmol) was dissolved in 25mL of ethanol, and a catalytic amount of concentrated sulfuric acid was added under stirring conditions, and heated to reflux for 12 hours. Cooled to room temperature, the reaction solution was concentrated on a rotary evaporator, and the obtained oil was diluted into 50 mL of ethyl acetate solution, and the organic phase was washed with saturated NaHCO 3 solution (25mL*2) and saturated brine (25mL), dried and filtered over anhydrous sodium sulfate, then concentrated by rotation to remove the solvent, and the solute was purified by column chromatography (mobile phase: petroleum ether: ethyl acetate = 10:1) to obtain Colorless oil D80 (1.1 g, 88.5%).
[0124] 1 H-NMR(300MHz,DMSO-d6)δ8.86(s,1H),8.77(s,1H),6.66-6.63(m,2H),6.47(dd,J=8.1,2.1Hz,1H),4.08 -3.99 (m, 2H), 3.42 (s, 2H), 1.20-1.14 (m, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com