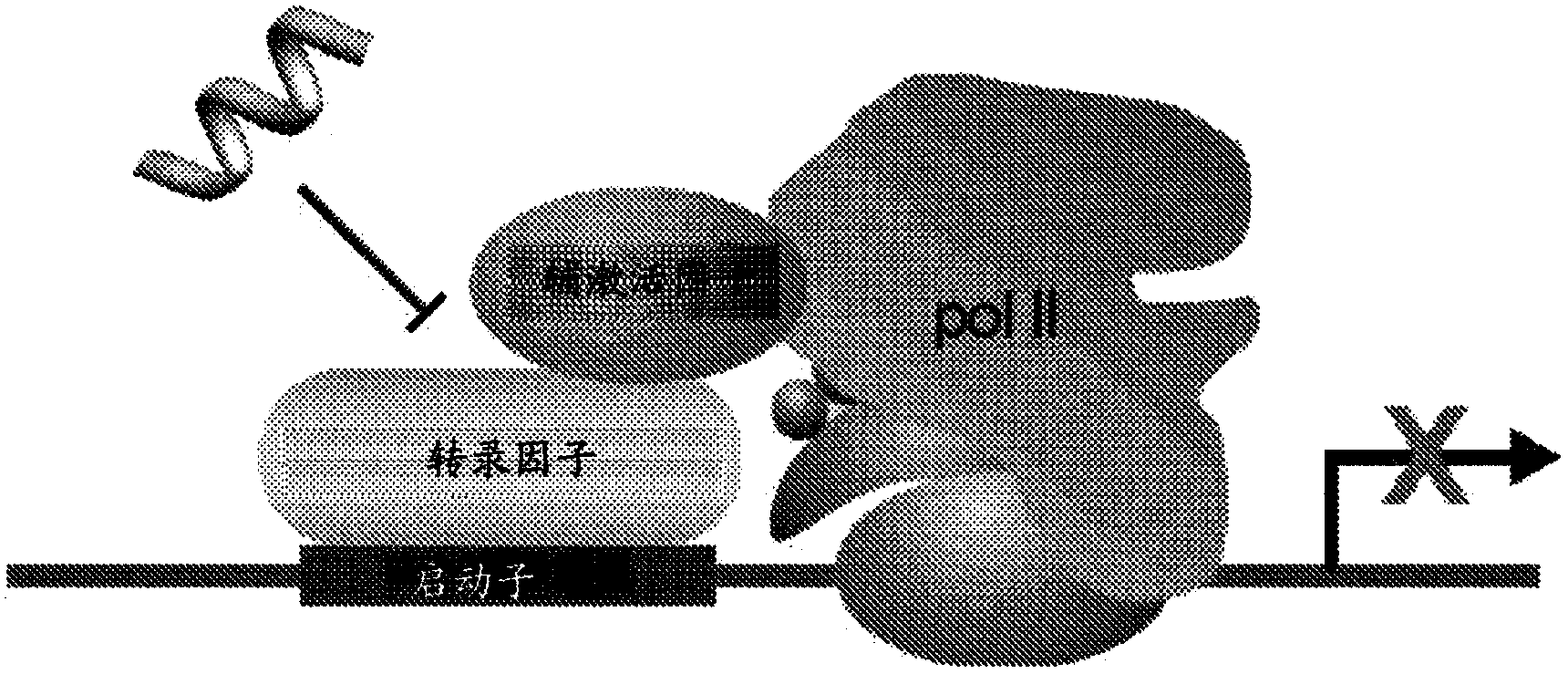
Control of hypoxia-inducible gene expression with oligooxopiperazine nonpeptidic helix mimetics
A technology of oligomeric oxopiperazines and pharmaceutical preparations, applied in the direction of using carriers to introduce foreign genetic material, medical preparations containing active ingredients, metabolic diseases, etc., and can solve problems such as leukocytosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0133] The synthesis of embodiment 1-oligomeric oxypiperazine
[0134] Oligomeric oxopiperazines were synthesized by solid phase synthesis as described in U.S. Patent No. 12 / 917,176 to Arora et al., which is hereby incorporated by reference in its entirety, as shown in the following scheme 1.
[0135]
[0136] plan 1
[0137] Dipeptide 1 was synthesized by standard Fmoc solid-phase peptide synthesis on Knorr resin in a solid-phase reaction vessel. The Fmoc group was removed by treatment with 20% piperidine in dimethylformamide (DMF), and the resin was washed sequentially with DMF, dichloromethane (DCM), methanol (MeOH) and diethyl ether, then dried in vacuo. o-Nitrobenzenesulfonyl chloride (Ns-Cl, 10 equiv) and chloropyridine (10 equiv) were dissolved in anhydrous DCM and added to the reaction vessel. The mixture was shaken at 23°C for 2 hours to give 2. The 2-containing resin was then washed sequentially with DMF, DCM, MeOH and diethyl ether, and dried under vacuum for...
Embodiment 2
[0140] Example 2 - MTT cell viability
[0141] Human breast cancer (MCF7) or human lung cancer (A549) cells were seeded in 96-well plates (Greiner) at a density of 5,000-10,000 cells in 200 μL fresh medium per well; MCF7 cells were seeded in 10% FBS (Irvine Scientific ) in RPMI medium (Gibco), A549 cells (ATCC) were seeded in F-12K medium (ATCC) with 2% FBS (Irvine Scientific). Then place the culture plate in the incubator (37°C, 5%-10% CO 2 ) until the desired confluency (approximately 70%) is reached (approximately 24-72 hours). Thereafter, the medium in all wells was replaced with a solution of BB2-125 or BB2-162 in the appropriate medium (150 μL for the 48 hour study and 200 μL for the 72 hour study, respectively). Then place the culture plate at 37°C, 5%-10% CO 2 maintained in the incubator for 48 or 72 hours. After 48 (or 72) hours of incubation with compounds, MTT (5 mg / ml in PBS, Sigma-Aldrich) (10% v / v) was added to each well and mixed carefully and thoroughly. P...
Embodiment 3
[0142] Example 3 - Luciferase Transcription Assay
[0143] All work was performed in a biohood with sterile supplies. MDA231-VEGF(HRE)-luc cells were seeded in 24-well plates (Greiner BioOne, 65,000 cells per well in 2 ml G418 DMEM medium (Gibco) with 10% FBS (Irvine Scientific)). Place the culture plate in the incubator for 24 hours (37°C and 5%-10% CO 2 ). Thereafter, individual wells were replaced with 10-μM or 20-μM solutions of BB2-125, BB2-162, BB2-164 or vehicle control (1 ml / well each) in medium (G418 DMEM, 10% FBS) Medium in. Incubate the culture plate for 6 hours (37°C, 5%-10% CO 2 ), then an aqueous solution of DFO (Sigma-Aldrich) (19.6 mg / ml, filtered through a 0.2 micron Tuffryn filter (PALL), 10 μL per well) was added to the appropriate wells to induce hypoxia. Incubate the plate for another 3 hours (37°C, 5%-10% CO 2 ), the appropriate culture plate was then placed in a plastic bag containing wet wipes and an anaerobic production pouch (BD GasPak EZ Pouch ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com